A Quinoxaline-Naphthaldehyde Conjugate for Colorimetric Determination of Copper Ion
- PMID: 35566259
- PMCID: PMC9105850
- DOI: 10.3390/molecules27092908
A Quinoxaline-Naphthaldehyde Conjugate for Colorimetric Determination of Copper Ion
Abstract
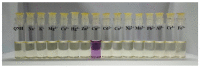
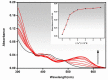
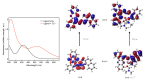
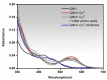
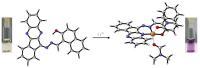
This work facilitates detection of bivalent copper ion by a simple Schiff base probe QNH based on a quinoxaline-naphthaldehyde framework. The detailed study in absorption spectroscopy and theoretical aspects and crystal study of the probe and probe-copper complex has been discussed. The detection limit of the probe in the presence of Cu2+ is 0.45 µM in HEPES-buffer/acetonitrile (3/7, v/v) medium for absorption study. The reversibility of the probe-copper complex has been investigated by EDTA. The selective visual detection of copper has been established also in gel form.
Keywords: Cu2+ colorimetric detection; ab initio calculations; absorption study; quinoxaline−naphthaldehyde conjugate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
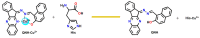
References
-
- Wang H., Zhu Q.-L., Zou R., Xu Q. Metal-organic frameworks for energy applications. Chem. 2017;2:52–80. doi: 10.1016/j.chempr.2016.12.002. - DOI
-
- Lai C.-H., Lu M.-Y., Chen L.-J. Metal sulfide nanostructures: Synthesis, properties and applications in energy conversion and storage. J. Mater. Chem. 2012;22:19–30. doi: 10.1039/C1JM13879K. - DOI
-
- Liu W., Yin X.-B. Metal–organic frameworks for electrochemical applications. Trends Anal. Chem. 2016;75:86–96. doi: 10.1016/j.trac.2015.07.011. - DOI
-
- Assche F.V., Clijsters H. Effects of metals on enzyme activity in plants. Plant Cell Environ. 1990;13:195–206. doi: 10.1111/j.1365-3040.1990.tb01304.x. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

