Opportunities and Challenges in Targeting the Proofreading Activity of SARS-CoV-2 Polymerase Complex
- PMID: 35566268
- PMCID: PMC9103157
- DOI: 10.3390/molecules27092918
Opportunities and Challenges in Targeting the Proofreading Activity of SARS-CoV-2 Polymerase Complex
Abstract
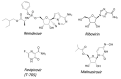
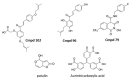
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the cause of the COVID-19 pandemic. While the development of vaccines and the emergence of antiviral therapeutics is promising, alternative strategies to combat COVID-19 (and potential future pandemics) remain an unmet need. Coronaviruses feature a unique mechanism that may present opportunities for therapeutic intervention: the RNA polymerase complex of coronaviruses is distinct in its ability to proofread and remove mismatched nucleotides during genome replication and transcription. The proofreading activity has been linked to the exonuclease (ExoN) activity of non-structural protein 14 (NSP14). Here, we review the role of NSP14, and other NSPs, in SARS-CoV-2 replication and describe the assays that have been developed to assess the ExoN function. We also review the nucleoside analogs and non-nucleoside inhibitors known to interfere with the proofreading activity of NSP14. Although not yet validated, the potential use of non-nucleoside proofreading inhibitors in combination with chain-terminating nucleosides may be a promising avenue for the development of anti-CoV agents.
Keywords: ExoN; NSP14; SARS-CoV-2; coronavirus; exonuclease; inhibitor; nucleoside; polymerase; proofreading.
Conflict of interest statement
J.D. is currently employed by Aligos Therapeutics, Inc. Z.A.G.-L. is currently employed by SAMDI Tech. The funder of the article had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- Lehmann K.C., Gulyaeva A., Zevenhoven-Dobbe J.C., Janssen G.M., Ruben M., Overkleeft H.S., van Veelen P.A., Samborskiy D.V., Kravchenko A.A., Leontovich A.M., et al. Discovery of an essential nucleotidylating activity associated with a newly delineated conserved domain in the RNA polymerase-containing protein of all nidoviruses. Nucleic Acids Res. 2015;43:8416–8434. doi: 10.1093/nar/gkv838. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

