Novasomes as Nano-Vesicular Carriers to Enhance Topical Delivery of Fluconazole: A New Approach to Treat Fungal Infections
- PMID: 35566287
- PMCID: PMC9103678
- DOI: 10.3390/molecules27092936
Novasomes as Nano-Vesicular Carriers to Enhance Topical Delivery of Fluconazole: A New Approach to Treat Fungal Infections
Abstract
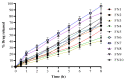
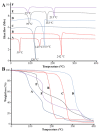
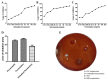
The occurrence of fungal infections has increased over the past two decades. It is observed that superficial fungal infections are treated by conventional dosage forms, which are incapable of treating deep infections due to the barrier activity possessed by the stratum corneum of the skin. This is why the need for a topical preparation with advanced penetration techniques has arisen. This research aimed to encapsulate fluconazole (FLZ) in a novasome in order to improve the topical delivery. The novasomes were prepared using the ethanol injection technique and characterized for percent entrapment efficiency (EE), particle size (PS), zeta potential (ZP), drug release, Fourier-transform infrared spectroscopy (FTIR), differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), scanning electron microscopy (SEM) and antifungal activity. The FN7 formulation with 94.45% EE, 110 nm PS and -24 ZP proved to be the best formulation. The FN7 formulation showed a 96% release of FLZ in 8 h. FTIR showed the compatibility of FLZ with excipients and DSC studies confirmed the thermal stability of FLZ in the developed formulation. The FN7 formulation showed superior inhibition of the growth of Candida albicans compared to the FLZ suspension using a resazurin reduction assay, suggesting high efficacy in inhibiting fungal growth.
Keywords: antifungal activity; ethanol injection technique; fluconazole; novasomes; toxicity study.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Use of Novasomes as a Vesicular Carrier for Improving the Topical Delivery of Terconazole: In Vitro Characterization, In Vivo Assessment and Exploratory Clinical Experimentation.Int J Nanomedicine. 2021 Jan 8;16:119-132. doi: 10.2147/IJN.S287383. eCollection 2021. Int J Nanomedicine. 2021. PMID: 33447031 Free PMC article. Clinical Trial.
-
Time to overcome fluconazole resistant Candida isolates: Solid lipid nanoparticles as a novel antifungal drug delivery system.Colloids Surf B Biointerfaces. 2016 Jun 1;142:400-407. doi: 10.1016/j.colsurfb.2016.03.013. Epub 2016 Mar 5. Colloids Surf B Biointerfaces. 2016. PMID: 26974361
-
Biodegradable Polymers-based Nanoparticles to Enhance the Antifungal Efficacy of Fluconazole against Candida albicans.Curr Pharm Biotechnol. 2022;23(5):749-757. doi: 10.2174/1389201022666210708105142. Curr Pharm Biotechnol. 2022. PMID: 34238149
-
New Generation of Fluconazole: A Review on Existing Researches and Technologies.Curr Drug Deliv. 2017;14(1):2-15. doi: 10.2174/1567201813666160502125620. Curr Drug Deliv. 2017. PMID: 27138299 Review.
-
A comprehensive review on recent nanosystems for enhancing antifungal activity of fenticonazole nitrate from different routes of administration.Drug Deliv. 2023 Dec;30(1):2179129. doi: 10.1080/10717544.2023.2179129. Drug Deliv. 2023. PMID: 36788709 Free PMC article. Review.
Cited by
-
Alginate as a Promising Biopolymer in Drug Delivery and Wound Healing: A Review of the State-of-the-Art.Int J Mol Sci. 2022 Aug 12;23(16):9035. doi: 10.3390/ijms23169035. Int J Mol Sci. 2022. PMID: 36012297 Free PMC article. Review.
-
Thyme Oil-Containing Fluconazole-Loaded Transferosomal Bigel for Transdermal Delivery.AAPS PharmSciTech. 2023 Nov 21;24(8):240. doi: 10.1208/s12249-023-02698-2. AAPS PharmSciTech. 2023. PMID: 37989918
-
Recent Advances of Chitosan Formulations in Biomedical Applications.Int J Mol Sci. 2022 Sep 19;23(18):10975. doi: 10.3390/ijms231810975. Int J Mol Sci. 2022. PMID: 36142887 Free PMC article. Review.
-
Development and Pharmacokinetic Evaluation of Novasomes for the Trans-nasal Delivery of Fluvoxamine Using Arachidonic Acid-Carboxymethyl Chitosan Conjugate.Pharmaceutics. 2023 Aug 31;15(9):2259. doi: 10.3390/pharmaceutics15092259. Pharmaceutics. 2023. PMID: 37765228 Free PMC article.
-
Nasal In-Situ Gels of Brij®-Enriched Novasomes as Optimistic Nanovesicular Carriers for Enhancing Anti-Depressant Action of Agomelatine.AAPS PharmSciTech. 2025 Apr 17;26(5):110. doi: 10.1208/s12249-025-03097-5. AAPS PharmSciTech. 2025. PMID: 40246739
References
-
- Ca A.A.R., Krishnan K., Sreelekshmi A., Arjun K., Nair S.C. Novasome: A pioneering advance-mentin vesicular drug delivery. Int. J. Appl. Pharm. 2021;13:59–64.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

