Investigating the Stability of Six Phenolic TMZ Ester Analogues, Incubated in the Presence of Porcine Liver Esterase and Monitored by HPLC
- PMID: 35566308
- PMCID: PMC9103334
- DOI: 10.3390/molecules27092958
Investigating the Stability of Six Phenolic TMZ Ester Analogues, Incubated in the Presence of Porcine Liver Esterase and Monitored by HPLC
Abstract
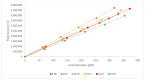
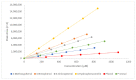
Previous published data from our group showed the encouraging in vitro activities of six phenolic temozolomide (TMZ) ester analogues (ES8-ES12 and ES14) with up to a five-fold increase in potency compared to TMZ against glioblastoma multiform cell lines and TMZ-resistant O6-methylguanine-DNA methyl transferase (MGMT)-positive primary cells. This study investigated the stabilities of the six phenolic TMZ ester analogues in the presence of porcine liver esterase (PLE) as a hydrolytic enzyme, using high-performance liquid chromatography (HPLC), monitored by a diode-array detector (DAD). Determining the rates of hydrolysis of the esters provided a useful insight into the feasibility of progressing them to the next phase of drug development. Fifty percent of TMZ esters consisting of para nitro, chloro, phenyl and tolyl groups (ES9, ES10, ES12 and ES14) were hydrolysed within the first 4.2 min of PLE exposure, while the TMZ esters consisting of para methoxy and nitrile groups (ES8 and ES11) demonstrated increased stability, with 50% hydrolysis achieved in 7.3 and 13.7 min, respectively. In conclusion, the survival of these phenolic TMZ esters on route to the target site of a brain tumor would be a challenge, mainly due to the undesirable rapid rate of hydrolysis. These findings therefore pose a question regarding the effectiveness of these esters in an in vivo setting.
Keywords: diode-array detector (DAD); glioblastoma multiform (GBM); high-performance liquid chromatography (HPLC); hydrolysis; phenolic TMZ esters; porcine liver esterase (PLE); stability; temozolomide (TMZ).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Up-Regulation of Cyclooxygenase-2 (COX-2) Expression by Temozolomide (TMZ) in Human Glioblastoma (GBM) Cell Lines.Int J Mol Sci. 2022 Jan 28;23(3):1545. doi: 10.3390/ijms23031545. Int J Mol Sci. 2022. PMID: 35163465 Free PMC article.
-
IKBKE enhances TMZ-chemoresistance through upregulation of MGMT expression in glioblastoma.Clin Transl Oncol. 2020 Aug;22(8):1252-1262. doi: 10.1007/s12094-019-02251-3. Epub 2019 Dec 21. Clin Transl Oncol. 2020. PMID: 31865606
-
SNAP reverses temozolomide resistance in human glioblastoma multiforme cells through down-regulation of MGMT.FASEB J. 2019 Dec;33(12):14171-14184. doi: 10.1096/fj.201901021RR. Epub 2019 Nov 7. FASEB J. 2019. PMID: 31725331
-
Alkylpurine-DNA-N-glycosylase confers resistance to temozolomide in xenograft models of glioblastoma multiforme and is associated with poor survival in patients.J Clin Invest. 2012 Jan;122(1):253-66. doi: 10.1172/JCI59334. Epub 2011 Dec 12. J Clin Invest. 2012. PMID: 22156195 Free PMC article.
-
Effect of lomeguatrib-temozolomide combination on MGMT promoter methylation and expression in primary glioblastoma tumor cells.Tumour Biol. 2013 Jun;34(3):1935-47. doi: 10.1007/s13277-013-0738-7. Epub 2013 Mar 22. Tumour Biol. 2013. PMID: 23519841
Cited by
-
Pig Liver Esterase Hydrolysis of 2-Arachidonoglycerol Exacerbates PRRSV-Induced Inflammation via PI3K-Akt-NF-κB Pathway.Cells. 2025 Aug 8;14(16):1227. doi: 10.3390/cells14161227. Cells. 2025. PMID: 40862706 Free PMC article.
-
Degradation kinetics of artesunate for the development of an ex-tempore intravenous injection.Malar J. 2022 Sep 6;21(1):256. doi: 10.1186/s12936-022-04278-4. Malar J. 2022. PMID: 36068561 Free PMC article.
References
-
- Lunt E., Newton C.G., Smith C., Stevens G.P., Stevens M.F., Straw C.G., Walsh R.J., Warren P.J., Fizames C., Lavelle F. Antitumor imidazotetrazines. 14. Synthesis and antitumor activity of 6- and 8-substituted imidazo [5,1-d]-1,2,3,5-tetrazinones and 8-substituted pyrazolo [5,1-d]-1,2,3,5-tetrazinones. J. Med. Chem. 1987;30:357–366. doi: 10.1021/jm00385a018. - DOI - PubMed
-
- Langnel D.A.F., Arrowsmith J., Stevens M.F.G. Antitumor imidazotetrazines. 38. New 8-substituted derivatives of the imidazo [5,1-d]-1,2,3,5-tetrazines temozolomide and mitozolomide. ARKIVOC. 2000;3:421–437. doi: 10.3998/ark.5550190.0001.324. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

