Direct Quantitation of Phytocannabinoids by One-Dimensional 1H qNMR and Two-Dimensional 1H-1H COSY qNMR in Complex Natural Mixtures
- PMID: 35566314
- PMCID: PMC9103933
- DOI: 10.3390/molecules27092965
Direct Quantitation of Phytocannabinoids by One-Dimensional 1H qNMR and Two-Dimensional 1H-1H COSY qNMR in Complex Natural Mixtures
Abstract
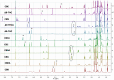
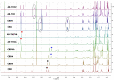
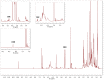
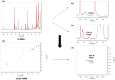
The widespread use of phytocannabinoids or cannabis extracts as ingredients in numerous types of products, in combination with the legal restrictions on THC content, has created a need for the development of new, rapid, and universal analytical methods for their quantitation that ideally could be applied without separation and standards. Based on previously described qNMR studies, we developed an expanded 1H qNMR method and a novel 2D-COSY qNMR method for the rapid quantitation of ten major phytocannabinoids in cannabis plant extracts and cannabis-based products. The 1H qNMR method was successfully developed for the quantitation of cannabidiol (CBD), cannabidiolic acid (CBDA), cannabinol (CBN), cannabichromene (CBC), cannabichromenic acid (CBCA), cannabigerol (CBG), cannabigerolic acid (CBGA), Δ9-tetrahydrocannabinol (Δ9-THC), Δ9-tetrahydrocannabinolic acid (Δ9-THCA), Δ8-tetrahydrocannabinol (Δ8-THC), cannabielsoin (CBE), and cannabidivarin (CBDV). Moreover, cannabidivarinic acid (CBDVA) and Δ9-tetrahydrocannabivarinic acid (Δ9-THCVA) can be distinguished from CBDA and Δ9-THCA respectively, while cannabigerovarin (CBGV) and Δ8-tetrahydrocannabivarin (Δ8-THCV) present the same 1H-spectra as CBG and Δ8-THC, respectively. The COSY qNMR method was applied for the quantitation of CBD, CBDA, CBN, CBG/CBGA, and THC/THCA. The two methods were applied for the analysis of hemp plants; cannabis extracts; edible cannabis medium-chain triglycerides (MCT); and hemp seed oils and cosmetic products with cannabinoids. The 1H-NMR method does not require the use of reference compounds, and it requires only a short time for analysis. However, complex extracts in 1H-NMR may have a lot of signals, and quantitation with this method is often hampered by peak overlap, with 2D NMR providing a solution to this obstacle. The most important advantage of the COSY NMR quantitation method was the determination of the legality of cannabis plants, extracts, and edible oils based on their THC/THCA content, particularly in the cases of some samples for which the determination of THC/THCA content by 1H qNMR was not feasible.
Keywords: COSY NMR; cannabichromene (CBC); cannabidiol (CBD); cannabielsoin (CBE); cannabigerol (CBG); cannabinoids; cannabinol (CBN); nuclear magnetic resonance spectroscopy (NMR); qNMR; tetrahydrocannabinol (THC).
Conflict of interest statement
One of the authors (V.M.) is owner of the Ekati Alchemy Lab, SL. The other authors declare no conflict of interest.
Figures

References
-
- Morales P., Hurst D.P., Reggio P.H. Molecular Targets of the Phytocannabinoids: Unraveling the Complex Chemistry and Pharmacology of Cannabis Sativa. In: Kinghorn A.D., Falk H., Gibbons S., Kobayashi J., editors. Progress in the Chemistry of Organic Natural Products. Springer International Publishing; Cham, Switzerland: 2017.
-
- Aizpurua-Olaizola O., Omar J., Navarro P., Olivares M., Etxebarria N., Usobiaga A. Identification and Quantification of Cannabinoids in Cannabis Sativa L. Plants by High Performance Liquid Chromatography-Mass Spectrometry. Anal. Bioanal. Chem. 2014;406:7549–7560. doi: 10.1007/s00216-014-8177-x. - DOI - PubMed
-
- Brighenti V., Pellati F., Steinbach M., Maran D., Benvenuti S. Development of a New Extraction Technique and HPLC Method for the Analysis of Non-Psychoactive Cannabinoids in Fibre-Type Cannabis Sativa L. (Hemp) J. Pharm. Biomed. Anal. 2017;143:228–236. doi: 10.1016/j.jpba.2017.05.049. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

