Zinc and Copper Ions Induce Aggregation of Human β-Crystallins
- PMID: 35566320
- PMCID: PMC9105653
- DOI: 10.3390/molecules27092970
Zinc and Copper Ions Induce Aggregation of Human β-Crystallins
Abstract
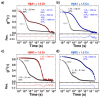
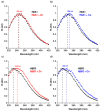
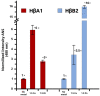
Cataracts are defined as the clouding of the lens due to the formation of insoluble protein aggregates. Metal ions exposure has been recognized as a risk factor in the cataract formation process. The γ and β crystallins are members of a larger family and share several structural features. Several studies have shown that copper and zinc ions induce the formation of γ-crystallins aggregates. However, the interaction of metal ions with β-crystallins, some of the most abundant crystallins in the lens, has not been explored until now. Here, we evaluate the effect of Cu(II) and Zn(II) ions on the aggregation of HβA1, as a representative of the acidic form, and HβB2, as a representative of the basic β-crystallins. We used several biophysical techniques and computational methods to show that Cu(II) and Zn(II) induce aggregation following different pathways. Both metal ions destabilize the proteins and impact protein folding. Copper induced a small conformational change in HβA1, leading to high-molecular-weight light-scattering aggregates, while zinc is more aggressive towards HβB2 and induces a larger conformational change. Our work provides information on the mechanisms of metal-induced aggregation of β-crystallins.
Keywords: cataracts; copper; crystallins; human beta crystallins; zinc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Copper and Zinc Ions Specifically Promote Nonamyloid Aggregation of the Highly Stable Human γ-D Crystallin.ACS Chem Biol. 2016 Jan 15;11(1):263-72. doi: 10.1021/acschembio.5b00919. Epub 2015 Dec 3. ACS Chem Biol. 2016. PMID: 26579725
-
Copper Reductase Activity and Free Radical Chemistry by Cataract-Associated Human Lens γ-Crystallins.J Am Chem Soc. 2023 Mar 29;145(12):6781-6797. doi: 10.1021/jacs.2c13397. Epub 2023 Mar 14. J Am Chem Soc. 2023. PMID: 36918380
-
Mercury-induced aggregation of human lens γ-crystallins reveals a potential role in cataract disease.J Biol Inorg Chem. 2018 Oct;23(7):1105-1118. doi: 10.1007/s00775-018-1607-z. Epub 2018 Aug 30. J Biol Inorg Chem. 2018. PMID: 30167892
-
Lens β-crystallins: the role of deamidation and related modifications in aging and cataract.Prog Biophys Mol Biol. 2014 Jul;115(1):21-31. doi: 10.1016/j.pbiomolbio.2014.02.004. Epub 2014 Mar 6. Prog Biophys Mol Biol. 2014. PMID: 24613629 Free PMC article. Review.
-
The βγ-crystallins: native state stability and pathways to aggregation.Prog Biophys Mol Biol. 2014 Jul;115(1):32-41. doi: 10.1016/j.pbiomolbio.2014.05.002. Epub 2014 May 14. Prog Biophys Mol Biol. 2014. PMID: 24835736 Free PMC article. Review.
Cited by
-
Mini-αA-crystallin protects a client lens protein from catastrophic aggregation due to heat stress.Protein Sci. 2025 Jul;34(7):e70199. doi: 10.1002/pro.70199. Protein Sci. 2025. PMID: 40545728
-
Characterization of the Interaction of Human γS Crystallin with Metal Ions and Its Effect on Protein Aggregation.Biomolecules. 2024 Dec 21;14(12):1644. doi: 10.3390/biom14121644. Biomolecules. 2024. PMID: 39766351 Free PMC article.
-
Combined Dextran-Graft-Polyacrylamide/Zinc Oxide Nanocarrier for Effective Anticancer Therapy in vitro.Int J Nanomedicine. 2023 Aug 28;18:4821-4838. doi: 10.2147/IJN.S416046. eCollection 2023. Int J Nanomedicine. 2023. PMID: 37662686 Free PMC article.
-
The Functional Significance of High Cysteine Content in Eye Lens γ-Crystallins.Biomolecules. 2024 May 17;14(5):594. doi: 10.3390/biom14050594. Biomolecules. 2024. PMID: 38786000 Free PMC article. Review.
-
Selenium intake help prevent age-related cataract formation: Evidence from NHANES 2001-2008.Front Nutr. 2023 Jan 27;10:1042893. doi: 10.3389/fnut.2023.1042893. eCollection 2023. Front Nutr. 2023. PMID: 36776608 Free PMC article.
References
-
- Bourne R.R.A., Steinmetz J.D., Saylan M., Mersha A.M., Weldemariam A.H., Wondmeneh T.G., Sreeramareddy C.T., Pinheiro M., Yaseri M., Yu C., et al. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: The Right to Sight: An analysis for the Global Burden of Disease Study. Lancet Glob. Health. 2021;9:e144–e160. doi: 10.1016/S2214-109X(20)30489-7. - DOI - PMC - PubMed
-
- Bourne R.R.A., Steinmetz J.D., Flaxman S., Briant P.S., Taylor H.R., Resnikoff S., Casson R.J., Abdoli A., Abu-Gharbieh E., Afshin A., et al. Trends in prevalence of blindness and distance and near vision impairment over 30 years: An analysis for the Global Burden of Disease Study. Lancet Glob. Health. 2021;9:e130–e143. doi: 10.1016/S2214-109X(20)30425-3. - DOI - PMC - PubMed
-
- Harshal W., Rishi G., Niraj P., Rajendra B., Anand G., Tushar P., Tirthajit B. A Comprehensive Review of Molecular Biology and Genetics of Cataract. Int. J. Res. Appl. Sci. Biotechnol. 2021;8:11–20. doi: 10.31033/ijrasb.8.2.2. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

