The Role of the Nucleotides in the Insertion of the bis-Molybdopterin Guanine Dinucleotide Cofactor into apo-Molybdoenzymes
- PMID: 35566344
- PMCID: PMC9103625
- DOI: 10.3390/molecules27092993
The Role of the Nucleotides in the Insertion of the bis-Molybdopterin Guanine Dinucleotide Cofactor into apo-Molybdoenzymes
Abstract
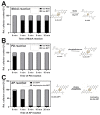
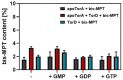
The role of the GMP nucleotides of the bis-molybdopterin guanine dinucleotide (bis-MGD) cofactor of the DMSO reductase family has long been a subject of discussion. The recent characterization of the bis-molybdopterin (bis-Mo-MPT) cofactor present in the E. coli YdhV protein, which differs from bis-MGD solely by the absence of the nucleotides, now enables studying the role of the nucleotides of bis-MGD and bis-MPT cofactors in Moco insertion and the activity of molybdoenzymes in direct comparison. Using the well-known E. coli TMAO reductase TorA as a model enzyme for cofactor insertion, we were able to show that the GMP nucleotides of bis-MGD are crucial for the insertion of the bis-MGD cofactor into apo-TorA.
Keywords: TMAO reductase; bis-MGD; chaperone; molybdenum cofactor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Reconstitution of Molybdoenzymes with Bis-Molybdopterin Guanine Dinucleotide Cofactors.Methods Mol Biol. 2019;1876:141-152. doi: 10.1007/978-1-4939-8864-8_9. Methods Mol Biol. 2019. PMID: 30317479
-
Identification of a bis-molybdopterin intermediate in molybdenum cofactor biosynthesis in Escherichia coli.J Biol Chem. 2013 Oct 11;288(41):29736-45. doi: 10.1074/jbc.M113.497453. Epub 2013 Sep 3. J Biol Chem. 2013. PMID: 24003231 Free PMC article.
-
Modulating the Molybdenum Coordination Sphere of Escherichia coli Trimethylamine N-Oxide Reductase.Biochemistry. 2018 Feb 20;57(7):1130-1143. doi: 10.1021/acs.biochem.7b01108. Epub 2018 Jan 31. Biochemistry. 2018. PMID: 29334455
-
Molybdenum enzymes, their maturation and molybdenum cofactor biosynthesis in Escherichia coli.Biochim Biophys Acta. 2013 Aug-Sep;1827(8-9):1086-101. doi: 10.1016/j.bbabio.2012.11.007. Epub 2012 Nov 29. Biochim Biophys Acta. 2013. PMID: 23201473 Review.
-
The biosynthesis of the molybdenum cofactors in Escherichia coli.Environ Microbiol. 2020 Jun;22(6):2007-2026. doi: 10.1111/1462-2920.15003. Epub 2020 Apr 6. Environ Microbiol. 2020. PMID: 32239579 Review.
Cited by
-
The History of the Molybdenum Cofactor-A Personal View.Molecules. 2022 Aug 3;27(15):4934. doi: 10.3390/molecules27154934. Molecules. 2022. PMID: 35956883 Free PMC article. Review.
-
Purification and Electron Transfer from Soluble c-Type Cytochrome TorC to TorA for Trimethylamine N-Oxide Reduction.Int J Mol Sci. 2024 Dec 12;25(24):13331. doi: 10.3390/ijms252413331. Int J Mol Sci. 2024. PMID: 39769096 Free PMC article.
-
Draft genome sequences of 12 Vibrio Harveyi clade isolates from Hong Kong coastal waters.Microbiol Resour Announc. 2025 Jun 12;14(6):e0023125. doi: 10.1128/mra.00231-25. Epub 2025 May 19. Microbiol Resour Announc. 2025. PMID: 40387472 Free PMC article.
-
Riboflavin kinase and pyridoxine 5'-phosphate oxidase complex formation envisages transient interactions for FMN cofactor delivery.Front Mol Biosci. 2023 Mar 28;10:1167348. doi: 10.3389/fmolb.2023.1167348. eCollection 2023. Front Mol Biosci. 2023. PMID: 37056721 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

