Simple and Expedient Access to Novel Fluorinated Thiazolo- and Oxazolo[3,2- a]pyrimidin-7-one Derivatives and Their Functionalization via Palladium-Catalyzed Reactions
- PMID: 35566366
- PMCID: PMC9100779
- DOI: 10.3390/molecules27093013
Simple and Expedient Access to Novel Fluorinated Thiazolo- and Oxazolo[3,2- a]pyrimidin-7-one Derivatives and Their Functionalization via Palladium-Catalyzed Reactions
Abstract
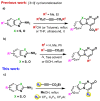
An efficient, versatile, and one-pot method for the preparation of novel fluorinated thiazolo- and oxazolo[3,2-a]pyrimidin-7-ones is described from 2-aminothiazoles or 2-amino-oxazoles and fluorinated alkynoates. This transformation, performed under transition-metal-free conditions, offers new fluorinated cyclized products with good to excellent yields. Moreover, the functionalization of these N-fused scaffolds via the Suzuki-Miyaura and Sonogashira cross-coupling reactions led to the synthesis of highly diverse thiazolo- and oxazolo[3,2-a]pyrimidin-7-ones.
Keywords: Sonogashira coupling; Suzuki-Miyaura; fluorinated alkynes; one-pot reaction; oxazolo[3,2-a]pyrimidin-7-ones; regioselective; thiazolo[3,2-a]pyrimidin-7-ones.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Sawant R.L., Ramdin S.S., Wadekar J.B. Synthesis, QSAR and docking studies of 5HT2A receptor antagonising thiazolo[3,2-a]pyrimidines as antipsychotic agents. Marmara Pharm. J. 2014;18:109–119. doi: 10.12991/mpj.2014187237. - DOI
-
- Selvam T.P., Karthick V., Kumar P.V., Ali M.A. Synthesis and structure-activity relationship study of 2-(substituted benzylidene)-7-(4-fluorophenyl)-5-(furan-2-yl)-2H-thiazolo[3,2-a]pyrimidin-3(7H)-one derivatives as anticancer agents. Drug Discov. Ther. 2012;6:198–204. doi: 10.5582/ddt.2012.v6.4.198. - DOI - PubMed
-
- Tozkoparan B., Ertan M., Krebs B., Läge M., Kelicen P., Demirdamar R. Condensed Heterocyclic Compounds: Synthesis and Antiinflammatory Activity of Novel Thiazolo[3,2-a]pyrimidines. Arch. Pharm. Pharm. Med. Chem. 1998;331:201–206. doi: 10.1002/(SICI)1521-4184(199806)331:6<201::AID-ARDP201>3.0.CO;2-T. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

