Production, Bioprocessing and Anti-Proliferative Activity of Camptothecin from Penicillium chrysogenum, "An Endozoic of Marine Sponge, Cliona sp.", as a Metabolically Stable Camptothecin Producing Isolate
- PMID: 35566384
- PMCID: PMC9104752
- DOI: 10.3390/molecules27093033
Production, Bioprocessing and Anti-Proliferative Activity of Camptothecin from Penicillium chrysogenum, "An Endozoic of Marine Sponge, Cliona sp.", as a Metabolically Stable Camptothecin Producing Isolate
Abstract
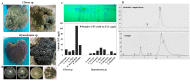
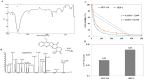
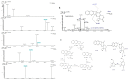
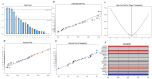
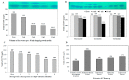
Exploring the metabolic potency of fungi as camptothecin producers raises the hope of their usage as an industrial source of camptothecin, due to their short-life span and the feasibility of metabolic engineering. However, the tiny yield and loss of camptothecin productivity of fungi during storage and sub-culturing are challenges that counteract this approach. Marine fungi could be a novel source for camptothecin production, with higher yield and reliable metabolic sustainability. The marine fungal isolate Penicillium chrysogenum EFBL # OL597937.1 derived from the sponge "Cliona sp." has been morphologically identified and molecularly confirmed, based on the Internal Transcribed Spacer sequence, exhibiting the highest yield of camptothecin (110 μg/L). The molecular structure and chemical identity of P. chrysogenum derived camptothecin has been resolved by HPLC, FTIR and LC-MS/MS analyses, giving the same spectroscopic profiles and mass fragmentation patterns as authentic camptothecin. The extracted camptothecin displayed a strong anti-proliferative activity towards HEP-2 and HCT-116 (IC50 values 0.33-0.35 µM). The yield of camptothecin was maximized by nutritional optimization of P. chrysogenum with a Plackett-Burman design, and the productivity of camptothecin increased by 1.8 fold (200 µg/L), compared to control fungal cultures. Upon storage at 4 °C as slope culture for 8 months, the productivity of camptothecin for P. chrysogenum was reduced by 40% compared to the initial culture. Visual fading of the mycelial pigmentation of P. chrysogenum was observed during fungal storage, matched with loss of camptothecin productivity. Methylene chloride extracts of Cliona sp. had the potency to completely restore the camptothecin productivity of P. chrysogenum, ensuring the partial dependence of the expression of the camptothecin biosynthetic machinery of P. chrysogenum on the chemical signals derived from the sponge, or the associated microbial flora. This is the first report describing the feasibility of P. chrysogenum, endozoic of Cliona sp., for camptothecin production, along with reliable metabolic biosynthetic stability, which could be a new platform for scaling-up camptothecin production.
Keywords: Cliona sp.; LC-MS/MS; Penicillium chrysogenum; anticancer activity; camptothecin.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Wall M.E., Wani M.C., Cook C.E., Palmer K.H., McPhail A.T., Sim G.A. Plant Antitumor Agents. I. The Isolation and Structure of Camptothecin, a Novel Alkaloidal Leukemia and Tumor Inhibitor from Camptotheca acuminata1,2. J. Am. Chem. Soc. 1966;88:3888–3890. doi: 10.1021/ja00968a057. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

