Effectiveness of Tocilizumab in Patients with Severe or Critical Lung Involvement in COVID-19: A Retrospective Study
- PMID: 35566412
- PMCID: PMC9101084
- DOI: 10.3390/jcm11092286
Effectiveness of Tocilizumab in Patients with Severe or Critical Lung Involvement in COVID-19: A Retrospective Study
Abstract
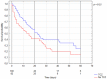
Introduction: Acute lung injury is associated with dysfunctional immune response to SARS-CoV-2. This leads to CRS, which require immunomodulatory treatments aiming to limit the excessive production of cytokines. The literature so far indicates the effectiveness of tocilizumab in patients with COVID-19-associated pneumonia, but there is no clear evidence of its effectiveness in patients with at least 50% lung involvement; therefore, we aimed to bridge this gap in knowledge. Materials and methods: Longitudinal data for 4287 patients with confirmed COVID-19 infection were collected between 1st March 2020 and 16th of January 2022. In total, 182 cases with lung involvement >50% and biochemical indicators of cytokine release storm (Il-6 >100 pg/mL) were selected and analyzed using non-parametric statistics and multivariate Cox models. Results: Among the 182 included patients, 100 (55%) were treated with TCZ, while 82 (45%) did not receive TCZ. The groups were balanced regarding demographics, lung involvement and biochemical markers. Overall mortality in the group was 63.1%. Mortality in the TCZ group was 58.0% compared to 69.5% (n = 57) in the non-TCZ group (p = 0.023). In multivariate Cox proportional hazards models, intravenous administration of tocilizumab was associated with lower probability of ICU admission (HR: 0333 (CI: 0.159−0.700, p = 0.004)) and lower mortality (HR: 0.57306 (CI: 0.354−0.927, p = 0.023)). Conclusions: Tocilizumab is effective as a treatment in the most severely ill patients, in whom the level of lung involvement by the inflammatory process can exceed 50% with coexisting biochemical indices of cytokine storm (Il-6 > 100 pg/mL).
Keywords: COVID-19; CRS; ICU; SARS-CoV-2; dexamethasone; mortality; tocilizumab.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Tocilizumab Use among Patients Who Developed Pulmonary Embolism in the Course of Cytokine Release Storm and COVID-19 Pneumonia-A Retrospective Study.Biomedicines. 2022 Jul 2;10(7):1581. doi: 10.3390/biomedicines10071581. Biomedicines. 2022. PMID: 35884886 Free PMC article.
-
The role of tocilizumab therapy in critically ill patients with severe acute respiratory syndrome coronavirus 2.J Osteopath Med. 2021 Jul 12;121(8):705-714. doi: 10.1515/jom-2020-0292. J Osteopath Med. 2021. PMID: 34237804
-
Benefits of Tocilizumab in patients with a severe form of SARS-CoV-2 infection: Experience of the intensive care unit of the Mohammed VI university hospital, Oujda.Ann Med Surg (Lond). 2021 Jul;67:102514. doi: 10.1016/j.amsu.2021.102514. Epub 2021 Jun 25. Ann Med Surg (Lond). 2021. PMID: 34221397 Free PMC article.
-
Tocilizumab in SARS-CoV-2 Patients with the Syndrome of Cytokine Storm: A Narrative Review.Rev Recent Clin Trials. 2021;16(2):138-145. doi: 10.2174/1574887115666200917110954. Rev Recent Clin Trials. 2021. PMID: 32940187 Review.
-
Mortality in tocilizumab-treated patients with COVID-19: a systematic review and meta-analysis.Clin Exp Rheumatol. 2020 Nov-Dec;38(6):1247-1254. Epub 2020 Dec 3. Clin Exp Rheumatol. 2020. PMID: 33275094
Cited by
-
Remdesivir Reduces Mortality in Hemato-Oncology Patients with COVID-19.J Inflamm Res. 2022 Aug 25;15:4907-4920. doi: 10.2147/JIR.S378347. eCollection 2022. J Inflamm Res. 2022. PMID: 36046662 Free PMC article.
-
Clinical Consequences for Individuals Treated with Tocilizumab for Serious COVID-19 Infection.Healthcare (Basel). 2023 Feb 17;11(4):607. doi: 10.3390/healthcare11040607. Healthcare (Basel). 2023. PMID: 36833140 Free PMC article.
-
Tocilizumab Use among Patients Who Developed Pulmonary Embolism in the Course of Cytokine Release Storm and COVID-19 Pneumonia-A Retrospective Study.Biomedicines. 2022 Jul 2;10(7):1581. doi: 10.3390/biomedicines10071581. Biomedicines. 2022. PMID: 35884886 Free PMC article.
-
Improved survival in intensive care unit in severe COVID-19 associated with amantadine use - retrospective study.Int J Infect Dis. 2022 Nov;124:143-151. doi: 10.1016/j.ijid.2022.09.026. Epub 2022 Sep 21. Int J Infect Dis. 2022. PMID: 36152957 Free PMC article.
-
Application of Machine Learning in Hospitalized Patients with Severe COVID-19 Treated with Tocilizumab.J Clin Med. 2022 Aug 12;11(16):4729. doi: 10.3390/jcm11164729. J Clin Med. 2022. PMID: 36012968 Free PMC article.
References
-
- WHO Coronavirus (COVID-19) Dashboard|WHO Coronavirus (COVID-19) Dashboard with Vaccination Data. [(accessed on 12 March 2022)]. Available online: https://covid19.who.int/
-
- COVID-19: Epidemiology, Clinical Features, and Prognosis of the Critically Ill Adult—Up To Date. [(accessed on 26 March 2022)]. Available online: https://www.uptodate.com/contents/COVID-19-epidemiology-clinical-feature....
LinkOut - more resources
Full Text Sources
Miscellaneous