New Advanced Imaging Parameters and Biomarkers-A Step Forward in the Diagnosis and Prognosis of TTR Cardiomyopathy
- PMID: 35566485
- PMCID: PMC9101617
- DOI: 10.3390/jcm11092360
New Advanced Imaging Parameters and Biomarkers-A Step Forward in the Diagnosis and Prognosis of TTR Cardiomyopathy
Abstract
Transthyretin amyloid cardiomyopathy (ATTR-CM) is an infiltrative disorder characterized by extracellular myocardial deposits of amyloid fibrils, with poor outcome, leading to heart failure and death, with significant treatment expenditure. In the era of a novel therapeutic arsenal of disease-modifying agents that target a myriad of pathophysiological mechanisms, timely and accurate diagnosis of ATTR-CM is crucial. Recent advances in therapeutic strategies shown to be most beneficial in the early stages of the disease have determined a paradigm shift in the screening, diagnostic algorithm, and risk classification of patients with ATTR-CM. The aim of this review is to explore the utility of novel specific non-invasive imaging parameters and biomarkers from screening to diagnosis, prognosis, risk stratification, and monitoring of the response to therapy. We will summarize the knowledge of the most recent advances in diagnostic, prognostic, and treatment tailoring parameters for early recognition, prediction of outcome, and better selection of therapeutic candidates in ATTR-CM. Moreover, we will provide input from different potential pathways involved in the pathophysiology of ATTR-CM, on top of the amyloid deposition, such as inflammation, endothelial dysfunction, reduced nitric oxide bioavailability, oxidative stress, and myocardial fibrosis, and their diagnostic, prognostic, and therapeutic implications.
Keywords: PET; SPECT; TTR amyloidosis; cardiac magnetic resonance; cardiac scintigraphy; new biomarkers; prognosis; speckle-tracking echocardiography.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
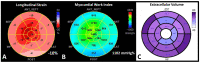


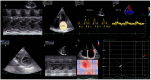



References
-
- Nitsche C., Scully P.R., Patel K.P., Kammerlander A.A., Koschutnik M., Dona C., Wollenweber T., Ahmed N., Thornton G.D., Kelion A.D., et al. Prevalence and Outcomes of Concomitant Aortic Stenosis and Cardiac Amyloidosis. J. Am. Coll. Cardiol. 2021;77:128–139. doi: 10.1016/j.jacc.2020.11.006. - DOI - PMC - PubMed
-
- Kittleson M.M., Maurer M.S., Ambardekar A.V., Bullock-Palmer R.P., Chang P.P., Eisen H.J., Nair A.P., Nativi-Nicolau J., Ruberg F.L. Cardiac Amyloidosis: Evolving Diagnosis and Management. A Scientific Statement Fron the American Heart Association. Circulation. 2020;142:e7–e22. doi: 10.1161/CIR.0000000000000792. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

