Ovocystatin Induced Changes in Expression of Alzheimer's Disease Relevant Proteins in APP/PS1 Transgenic Mice
- PMID: 35566501
- PMCID: PMC9103311
- DOI: 10.3390/jcm11092372
Ovocystatin Induced Changes in Expression of Alzheimer's Disease Relevant Proteins in APP/PS1 Transgenic Mice
Abstract
Background: Ovocystatin is marked by structural and biological similarities to human cystatin C, which plays an important role in the course of neurodegenerative diseases. Recently, it has been shown that ovocystatin might prevent aging-related cognitive impairment in rats and reduce memory decline in an APP/PS1 mice model. Thus, this study aimed to assess the effect of ovocystatin on histopathological changes in APP/PS1 mice. Materials and methods: Ovocystatin was administered intraperitoneally for four weeks (40 μg/mouse) to 35-weeks-old transgenic (AD, n = 14) and wild type (NCAR, n = 15) mice (stock B6C3-Tg(APPswe, PSEN1dE9)85Dbo/Mmjax). A histopathological evaluation comprised antibodies directed against β-amyloid (1:400, SIG-39320-1000, Covance) and Tau (1:4000, AHB0042, Invitrogen). Three regions of the hippocampus— the dentate gyrus (DG) and the cornu ammonis (CA1 and CA3)—were analyzed by immunohistochemistry in each animal. All differences are expressed as percentage relative to the control group. Results: The main results showed that the percentage of immunoreactive area of β-amyloid, tau protein deposits in APP/PS1+ovCYS was decreased in DG, CA1, and CA3 regions compared with the APP/PS1 control, respectively (p < 0.05). Conclusions: Ovocystatin caused significant changes in the expression pattern of all investigated proteins in hippocampal tissues both in APP/PS1 and NCAR mice.
Keywords: Alzheimer’s disease; chicken cystatin; cystatin C; mice; ovocystatin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
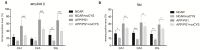

Similar articles
-
Beneficial effect of ovocystatin on the cognitive decline in APP/PS1 transgenic mice.Adv Med Sci. 2019 Mar;64(1):65-71. doi: 10.1016/j.advms.2018.08.002. Epub 2018 Nov 30. Adv Med Sci. 2019. PMID: 30504006
-
Four-month treadmill exercise prevents the decline in spatial learning and memory abilities and the loss of spinophilin-immunoreactive puncta in the hippocampus of APP/PS1 transgenic mice.Neurobiol Dis. 2020 Mar;136:104723. doi: 10.1016/j.nbd.2019.104723. Epub 2019 Dec 27. Neurobiol Dis. 2020. PMID: 31887353
-
Fluoxetine attenuates the impairment of spatial learning ability and prevents neuron loss in middle-aged APPswe/PSEN1dE9 double transgenic Alzheimer's disease mice.Oncotarget. 2017 Apr 25;8(17):27676-27692. doi: 10.18632/oncotarget.15398. Oncotarget. 2017. PMID: 28430602 Free PMC article.
-
Effect of running exercise on the number of the neurons in the hippocampus of young transgenic APP/PS1 mice.Brain Res. 2018 Aug 1;1692:56-65. doi: 10.1016/j.brainres.2018.04.033. Epub 2018 Apr 30. Brain Res. 2018. PMID: 29715445
-
Modeling Alzheimer's disease in transgenic mice: effect of age and of presenilin1 on amyloid biochemistry and pathology in APP/London mice.Exp Gerontol. 2000 Sep;35(6-7):831-41. doi: 10.1016/s0531-5565(00)00149-2. Exp Gerontol. 2000. PMID: 11053674 Review.
Cited by
-
Effect of Ovocystatin on Amyloid β 1-42 Aggregation-In Vitro Studies.Int J Mol Sci. 2023 Mar 12;24(6):5433. doi: 10.3390/ijms24065433. Int J Mol Sci. 2023. PMID: 36982505 Free PMC article.
-
LRP1 and RAGE Genes Transporting Amyloid and Tau Protein in the Hippocampal CA3 Area in an Ischemic Model of Alzheimer's Disease with 2-Year Survival.Cells. 2023 Dec 4;12(23):2763. doi: 10.3390/cells12232763. Cells. 2023. PMID: 38067191 Free PMC article.
-
Cystatins: unravelling the biological implications for neuroprotection.Arch Med Sci. 2023 Sep 19;20(1):157-166. doi: 10.5114/aoms/171706. eCollection 2024. Arch Med Sci. 2023. PMID: 38414464 Free PMC article.
References
-
- Eleti S. Drugs in Alzheimer’s disease Dementia: An overview of current pharmacological management and future directions. Psychiatr. Danub. 2016;28:136–140. - PubMed
-
- Ihl R., Bunevicius R., Frölich L., Winblad B., Schneider L.S., Dubois B., Burns A., Thibaut F., Kasper S., Möller H.-J., et al. World Federation of Societies of Biological Psychiatry guidelines for the pharmacological treatment of dementias in primary care. Int. J. Psychiatry Clin. Pract. 2014;19:2–7. doi: 10.3109/13651501.2014.961931. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

