Genetic Characteristics According to Subgroup of Acute Myeloid Leukemia with Myelodysplasia-Related Changes
- PMID: 35566503
- PMCID: PMC9105081
- DOI: 10.3390/jcm11092378
Genetic Characteristics According to Subgroup of Acute Myeloid Leukemia with Myelodysplasia-Related Changes
Abstract
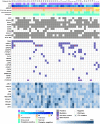
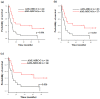
Acute myeloid leukemia with myelodysplasia-related changes (AML-MRC) includes heterogeneous conditions such as previous history and specific cytogenetic and morphological properties. In this study, we analyze genetic aberrations using an RNA-based next-generation sequencing (NGS) panel assay in 45 patients with AML-MRC and detect 4 gene fusions of KMT2A-SEPT9, KMT2A-ELL, NUP98-NSD1, and RUNX1-USP42 and 81 somatic mutations. Overall, all patients had genetic aberrations comprising of not only cytogenetic changes, but also gene fusions and mutations. We also demonstrated several characteristic genetic mutations according to the AML-MRC subgroup. TP53 was the most commonly mutated gene (n = 11, 24%) and all were found in the AML-MRC subgroup with myelodysplastic syndrome-defining cytogenetic abnormalities (AML-MRC-C) (p = 0.002). These patients showed extremely poor overall survival not only in AML-MRC, but also within the AML-MRC-C subgroup. The ASXL1 (n = 9, 20%) and SRSF2 (n = 7, 16%) mutations were associated with the AML-MRC subgroup with >50% dysplasia in at least two lineages (AML-MRC-M) and were frequently co-mutated (55%, 6/11, p < 0.001). Both mutations could be used as surrogate markers to diagnose AML-MRC, especially when the assessment of multilineage dysplasia was difficult. IDH1/IDH2 (n = 13, 29%) were most commonly mutated in AML-MRC, followed by CEBPA (n = 5, 11%), PTPN11 (n = 5, 11%), FLT3 (n = 4, 9%), IDH1 (n = 4, 9%), and RUNX1 (n = 4, 9%). These mutations were not limited in any AML-MRC subgroup and could have more significance as a risk factor or susceptibility marker for target therapy in not only AML-MRC, but also other AML categories.
Keywords: RNA-based next-generation sequencing; acute myeloid leukemia with myelodysplasia-related changes; morphological properties; previous history; specific cytogenetic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Arber D.A., Orazi A., Hasserjian R., Thiele J., Borowitz M.J., Le Beau M.M., Bloomfield C.D., Cazzola M., Vardiman J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. doi: 10.1182/blood-2016-03-643544. - DOI - PubMed
-
- Wandt H., Schäkel U., Kroschinsky F., Prange-Krex G., Mohr B., Thiede C., Pascheberg U., Soucek S., Schaich M., Ehninger G. MLD according to the WHO classification in AML has no correlation with age and no independent prognostic relevance as analyzed in 1766 patients. Blood. 2008;111:1855–1861. doi: 10.1182/blood-2007-08-101162. - DOI - PubMed
-
- Miesner M., Haferlach C., Bacher U., Weiss T., Macijewski K., Kohlmann A., Klein H.-U., Dugas M., Kern W., Schnittger S., et al. Multilineage dysplasia (MLD) in acute myeloid leukemia (AML) correlates with MDS-related cytogenetic abnormalities and a prior history of MDS or MDS/MPN but has no independent prognostic relevance: A comparison of 408 cases classified as “AML not otherwise specified” (AML-NOS) or “AML with myelodysplasia-related changes” (AML-MRC) Blood. 2010;116:2742–2751. - PubMed
-
- McGowan-Jordan J., Hastings R.J., Moore S. ISCN 2020. An International System for Human Cytogenomic Nomenclature (2020) Karger; Basel, Switzerland: 2020. p. 170.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

