A Comprehensive Update on Retinal Vasculitis: Etiologies, Manifestations and Treatments
- PMID: 35566655
- PMCID: PMC9101900
- DOI: 10.3390/jcm11092525
A Comprehensive Update on Retinal Vasculitis: Etiologies, Manifestations and Treatments
Abstract
Retinal vasculitis is characterized by inflammatory involvement of retinal arterioles, venules and/or capillaries and can be associated with a myriad of systemic and ophthalmic diseases. In this review, we have comprehensively discussed the etiologies, clinical manifestations, and presentations of retinal vasculitis. We have also included newer advances in imaging in retinal vasculitis such as OCTA and widefield imaging.
Keywords: eye; fluorescein angiography; ocular inflammation; retinal vasculitis; uveitis.
Conflict of interest statement
U.P. has served as principal investigator or consultant for: Abbvie, Alcon, Alimera, Allergan, Bayer, Dompé, Heidelberg-E., Janssen, Novartis, Pfizer, Santen, Shire, Thea, Winzer, Abbvie, Allergan, Lilly, Novartis, Santen, Thea and has received research support from BMBF, DOG, DFG, and the European Union. He holds patents (PCT/EP2019/066419, C75928WOUS) and shares of Novartis, BioNtec and Zeiss. A.R. received speaking honoria from Bayer Healthcare and Novartis and served as consultant for Novartis. A.R. received research support from Deutsche Forschungsgemeinschaft (DFG) (RU 2020/3-1) and is a participant in the BIH-Charité Clinician Scientist Program funded by Charité—Universitätsmedizin Berlin and the Berlin Institute of Health. A.A. has no conflict to declare.
Figures

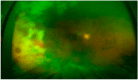

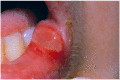
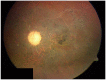
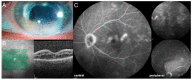
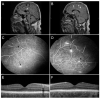
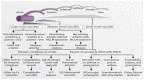
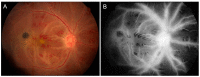
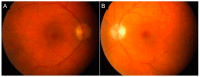
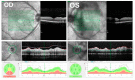
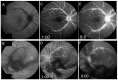
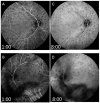
Similar articles
-
An Update on Noninfectious Retinal Vasculitis.Ophthalmologica. 2024 Jun 5:1-14. doi: 10.1159/000539608. Online ahead of print. Ophthalmologica. 2024. PMID: 38830340 Review.
-
RETINAL VASCULITIS, A COMMON MANIFESTATION OF IDIOPATHIC PEDIATRIC UVEITIS?Retina. 2021 Mar 1;41(3):610-619. doi: 10.1097/IAE.0000000000002885. Retina. 2021. PMID: 32658162
-
Evaluation of vascular changes in intermediate uveitis and retinal vasculitis using swept-source wide-field optical coherence tomography angiography.Br J Ophthalmol. 2019 Sep;103(9):1289-1295. doi: 10.1136/bjophthalmol-2018-313078. Epub 2018 Dec 11. Br J Ophthalmol. 2019. PMID: 30538102 Free PMC article.
-
Retinal vasculitis in rheumatic diseases: an unseen burden.Clin Rheumatol. 2013 Jan;32(1):7-13. doi: 10.1007/s10067-012-2078-1. Epub 2012 Sep 6. Clin Rheumatol. 2013. PMID: 22955636 Review.
-
Swept-source optical coherence tomography angiography of retinal occlusive vasculitis following brolucizumab administration: a case report.BMC Ophthalmol. 2022 Jun 3;22(1):244. doi: 10.1186/s12886-022-02465-0. BMC Ophthalmol. 2022. PMID: 35659269 Free PMC article.
Cited by
-
Successful treatment of idiopathic retinal vasculitis with rituximab in two patients.Am J Ophthalmol Case Rep. 2023 Apr 12;30:101844. doi: 10.1016/j.ajoc.2023.101844. eCollection 2023 Jun. Am J Ophthalmol Case Rep. 2023. PMID: 37124152 Free PMC article.
-
Neovascular Glaucoma as the First Symptom of Bilateral Occlusive Retinal Vasculitis in a 4-Year-Old Girl: A Case Report.Biomedicines. 2025 Jan 9;13(1):148. doi: 10.3390/biomedicines13010148. Biomedicines. 2025. PMID: 39857733 Free PMC article.
-
Optical coherence tomography angiography to assess for retinal vascular changes in Neuro-Sjögren.Ther Adv Ophthalmol. 2024 Oct 31;16:25158414241294024. doi: 10.1177/25158414241294024. eCollection 2024 Jan-Dec. Ther Adv Ophthalmol. 2024. PMID: 39493254 Free PMC article.
-
RETINAL VASCULITIS SEVERITY ASSESSMENT: Intraobserver and Interobserver Reliability of a New Scheme for Grading Wide-Field Fluorescein Angiograms in Retinal Vasculitis.Retina. 2023 Sep 1;43(9):1534-1543. doi: 10.1097/IAE.0000000000003838. Retina. 2023. PMID: 37229721 Free PMC article.
-
A systematic review of OCT and OCT angiography in retinal vasculitis.J Ophthalmic Inflamm Infect. 2023 Jan 30;13(1):1. doi: 10.1186/s12348-023-00327-4. J Ophthalmic Inflamm Infect. 2023. PMID: 36715778 Free PMC article. Review.
References
-
- Agarwal A., Karkhur S., Aggarwal K., Invernizzi A., Singh R., Dogra M.R., Gupta V., Gupta A., Do D.V., Nguyen Q.D. Epidemiology and clinical features of inflammatory retinal vascular occlusions: Pooled data from two tertiary-referral institutions. Clin. Exp. Ophthalmol. 2018;46:62–74. doi: 10.1111/ceo.12997. - DOI - PubMed
-
- Pelegrín L., Hernández-Rodríguez J., Espinosa G., Llorenç V., Sainz-De-La-Maza M., Fontenla J.R., Martínez J.A., Cid M.C., Adán A. Characterization of isolated retinal vasculitis. Analysis of a cohort from a single center and literature review. Autoimmun. Rev. 2017;16:237–243. doi: 10.1016/j.autrev.2017.01.006. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

