Effect of Matrix Size Reduction on Textural Information in Clinical Magnetic Resonance Imaging
- PMID: 35566657
- PMCID: PMC9103884
- DOI: 10.3390/jcm11092526
Effect of Matrix Size Reduction on Textural Information in Clinical Magnetic Resonance Imaging
Abstract
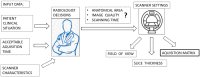
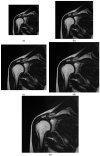
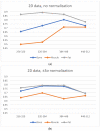
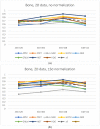
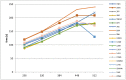
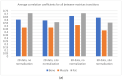
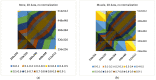
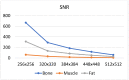
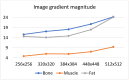
The selection of the matrix size is an important element of the magnetic resonance imaging (MRI) process, and has a significant impact on the acquired image quality. Signal to noise ratio, often used to assess MR image quality, has its limitations. Thus, for this purpose we propose a novel approach: the use of texture analysis as an index of the image quality that is sensitive for the change of matrix size. Image texture in biomedical images represents tissue and organ structures visualized via medical imaging modalities such as MRI. The correlation between texture parameters determined for the same tissues visualized in images acquired with different matrix sizes is analyzed to aid in the assessment of the selection of the optimal matrix size. T2-weighted coronal images of shoulders were acquired using five different matrix sizes while maintaining the same field of view; three regions of interest (bone, fat, and muscle) were considered. Lin's correlation coefficients were calculated for all possible pairs of the 310-element texture feature vectors evaluated for each matrix. The obtained results are discussed considering the image noise and blurring effect visible in images acquired with smaller matrices. Taking these phenomena into account, recommendations for the selection of the matrix size used for the MRI imaging were proposed.
Keywords: image quality; magnetic resonance; matrix size; texture analysis.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures










References
Grants and funding
LinkOut - more resources
Full Text Sources

