Serological Response to Three, Four and Five Doses of SARS-CoV-2 Vaccine in Kidney Transplant Recipients
- PMID: 35566691
- PMCID: PMC9105533
- DOI: 10.3390/jcm11092565
Serological Response to Three, Four and Five Doses of SARS-CoV-2 Vaccine in Kidney Transplant Recipients
Abstract
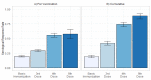
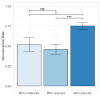
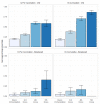
Mortality from COVID-19 among kidney transplant recipients (KTR) is high, and their response to three vaccinations against SARS-CoV-2 is strongly impaired. We retrospectively analyzed the serological response of up to five doses of the SARS-CoV-2 vaccine in KTR from 27 December 2020 until 31 December 2021. Particularly, the influence of the different dose adjustment regimens for mycophenolic acid (MPA) on serological response to fourth vaccination was analyzed. In total, 4277 vaccinations against SARS-CoV-2 in 1478 patients were analyzed. Serological response was 19.5% after 1203 basic immunizations, and increased to 29.4%, 55.6%, and 57.5% in response to 603 third, 250 fourth, and 40 fifth vaccinations, resulting in a cumulative response rate of 88.7%. In patients with calcineurin inhibitor and MPA maintenance immunosuppression, pausing MPA and adding 5 mg prednisolone equivalent before the fourth vaccination increased the serological response rate to 75% in comparison to the no dose adjustment (52%) or dose reduction (46%). Belatacept-treated patients had a response rate of 8.7% (4/46) after three vaccinations and 12.5% (3/25) after four vaccinations. Except for belatacept-treated patients, repeated SARS-CoV-2 vaccination of up to five times effectively induces serological response in kidney transplant recipients. It can be enhanced by pausing MPA at the time of vaccination.
Keywords: COVID-19; immunosuppression; kidney transplantation; vaccination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Reischig T., Kacer M., Vlas T., Drenko P., Kielberger L., Machova J., Topolcan O., Kucera R., Kormunda S. Insufficient response to mRNA SARS-CoV-2 vaccine and high incidence of severe COVID-19 in kidney transplant recipients during pandemic. Am. J. Transplant. 2021;22:801–812. doi: 10.1111/ajt.16902. - DOI - PMC - PubMed
-
- CDC CDC COVID Data Tracker. [(accessed on 28 January 2022)]; Available online: https://covid.cdc.gov/covid-data-tracker/#rates-by-vaccine-status.
LinkOut - more resources
Full Text Sources
Miscellaneous

