Urinary Stent Development and Evaluation Models: In Vitro, Ex Vivo and In Vivo-A European Network of Multidisciplinary Research to Improve Urinary Stents (ENIUS) Initiative
- PMID: 35566810
- PMCID: PMC9102855
- DOI: 10.3390/polym14091641
Urinary Stent Development and Evaluation Models: In Vitro, Ex Vivo and In Vivo-A European Network of Multidisciplinary Research to Improve Urinary Stents (ENIUS) Initiative
Abstract
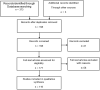
Background: When trying to modify urinary stents, certain pre-clinical steps have to be followed before clinical evaluation in humans. Usually, the process starts as an in silico assessment. The urinary tract is a highly complex, dynamic and variable environment, which makes a computer simulation closely reflecting physiological conditions extremely challenging. Therefore, the pre-clinical evaluation needs to go through further steps of in vitro, ex vivo and in vivo assessments. Methods and materials: Within the European Network of Multidisciplinary Research to Improve Urinary Stents (ENIUS), the authors summarized and evaluated stent assessment models in silico, in vitro, ex vivo and in vivo. The topic and relevant sub-topics were researched in a systematic literature search in Embase, Scope, Web of Science and PubMed. Clinicaltrials.gov was consulted for ongoing trials. Articles were selected systematically according to guidelines with non-relevant, non-complete, and non-English or Spanish language articles excluded. Results: In the first part of this paper, we critically evaluate in vitro stent assessment models used over the last five decades, outlining briefly their strengths and weaknesses. In the second part, we provide a step-by-step guide on what to consider when setting up an ex vivo model for stent evaluation on the example of a biodegradable stent. Lastly, the third part lists and discusses the pros and cons of available animal models for urinary stent evaluation, this being the final step before human trials. Conclusions: We hope that this overview can provide a practical guide and a critical discussion of the experimental pre-clinical evaluation steps needed, which will help interested readers in choosing the right methodology from the start of a stent evaluation process once an in silico assessment has been completed. Only a transparent multidisciplinary approach using the correct methodology will lead to a successful clinical implementation of any new or modified stent.
Keywords: animal models; design; encrustation; evaluation; ex vivo; in vitro; in vivo; material; urinary stent; urinary tract models.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




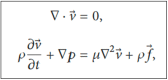
Similar articles
-
Potential strategies to prevent encrustations on urinary stents and catheters - thinking outside the box: a European network of multidisciplinary research to improve urinary stents (ENIUS) initiative.Expert Rev Med Devices. 2021 Jul;18(7):697-705. doi: 10.1080/17434440.2021.1939010. Epub 2021 Jun 11. Expert Rev Med Devices. 2021. PMID: 34085555
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
The Effectiveness of Integrated Care Pathways for Adults and Children in Health Care Settings: A Systematic Review.JBI Libr Syst Rev. 2009;7(3):80-129. doi: 10.11124/01938924-200907030-00001. JBI Libr Syst Rev. 2009. PMID: 27820426
-
Technology insight: Novel ureteral stent materials and designs.Nat Clin Pract Urol. 2004 Nov;1(1):44-8. doi: 10.1038/ncpuro0014. Nat Clin Pract Urol. 2004. PMID: 16474466 Review.
-
Advances in ureteral stent design.J Endourol. 2003 May;17(4):195-9. doi: 10.1089/089277903765444294. J Endourol. 2003. PMID: 12816579 Review.
Cited by
-
An Evaluation of Parylene Thin Films to Prevent Encrustation for a Urinary Bladder Pressure MEMS Sensor System.Polymers (Basel). 2023 Aug 27;15(17):3559. doi: 10.3390/polym15173559. Polymers (Basel). 2023. PMID: 37688185 Free PMC article.
-
Interactive Effects of Copper-Doped Urological Implants with Tissue in the Urinary Tract for the Inhibition of Cell Adhesion and Encrustation in the Animal Model Rat.Polymers (Basel). 2022 Aug 16;14(16):3324. doi: 10.3390/polym14163324. Polymers (Basel). 2022. PMID: 36015581 Free PMC article.
-
Advances in experimental bladder models: bridging the gap between in vitro and in vivo approaches for investigating urinary tract infections.BMC Urol. 2024 Sep 23;24(1):206. doi: 10.1186/s12894-024-01590-w. BMC Urol. 2024. PMID: 39313789 Free PMC article. Review.
-
Establishment of an artificial urine model in vitro and rat or pig model in vivo to evaluate urinary crystal adherence.Sci Rep. 2024 May 25;14(1):12001. doi: 10.1038/s41598-024-62766-w. Sci Rep. 2024. PMID: 38796538 Free PMC article.
References
-
- Tortora G.J., Derrickson B. Principles of Anatomy and Physiology. 11th ed. John Wiley & Sons; Hoboken, NJ, USA: 2006. pp. 933–1035.
-
- Leeson C.R., Leeson T.S., Paparo A.A. Text Book of Histology. 5th ed. W.B. Saunders; Philadelphia, PA, USA: 1985.
-
- Berne R.M., Levy M.N. Principles of Physiology. 3rd ed. Mosby Inc.; St Louis, NO, USA: 2000.
-
- Pocock G., Richards C.D. Human Physiology: The Basis of Medicine. 2nd ed. Oxford University Press; Oxford, UK: 2004.
-
- Atala A., Denstedt J. Biomaterials and Tissue Engineering in Urology. Woodhead Publishing Limited & CRC Press LLC; Sawston, UK: 2009.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

