Biocompatible Nanoparticles Based on Amphiphilic Random Polypeptides and Glycopolymers as Drug Delivery Systems
- PMID: 35566847
- PMCID: PMC9104652
- DOI: 10.3390/polym14091677
Biocompatible Nanoparticles Based on Amphiphilic Random Polypeptides and Glycopolymers as Drug Delivery Systems
Abstract
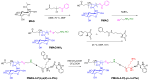
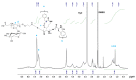
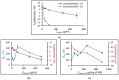
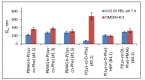
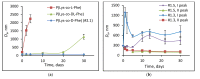
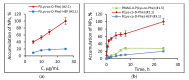
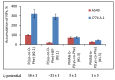
In this research, the development and investigation of novel nanoobjects based on biodegradable random polypeptides and synthetic non-degradable glycopolymer poly(2-deoxy-2-methacrylamido-d-glucose) were proposed as drug delivery systems. Two different approaches have been applied for preparation of such nanomaterials. The first one includes the synthesis of block-random copolymers consisting of polypeptide and glycopolymer and capable of self-assembly into polymer particles. The synthesis of copolymers was performed using sequential reversible addition-fragmentation chain transfer (RAFT) and ring-opening polymerization (ROP) techniques. Amphiphilic poly(2-deoxy-2-methacrylamido-d-glucose)-b-poly(l-lysine-co-l-phenylalanine) (PMAG-b-P(Lys-co-Phe)) copolymers were then used for preparation of self-assembled nanoparticles. Another approach for the formation of polypeptide-glycopolymer particles was based on the post-modification of preformed polypeptide particles with an oxidized glycopolymer. The conjugation of the polysaccharide on the surface of the particles was achieved by the interaction of the aldehyde groups of the oxidized glycopolymer with the amino groups of the polymer on particle surface, followed by the reduction of the formed Schiff base with sodium borohydride. A comparative study of polymer nanoparticles developed with its cationic analogues based on random P(Lys-co-d-Phe), as well as an anionic one-P(Lys-co-d-Phe) covered with heparin--was carried out. In vitro antitumor activity of novel paclitaxel-loaded PMAG-b-P(Lys-co-Phe)-based particles towards A549 (human lung carcinoma) and MCF-7 (human breast adenocarcinoma) cells was comparable to the commercially available Paclitaxel-LANS.
Keywords: amphiphilic copolymers; cellular uptake of particles; drug delivery systems; polymer particles; polypeptides; random and block-random copolymers; synthetic glycopolymers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

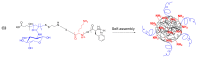
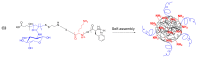


References
Grants and funding
LinkOut - more resources
Full Text Sources

