A Degradable Difunctional Initiator for ATRP That Responds to Hydrogen Peroxide
- PMID: 35566902
- PMCID: PMC9099818
- DOI: 10.3390/polym14091733
A Degradable Difunctional Initiator for ATRP That Responds to Hydrogen Peroxide
Abstract
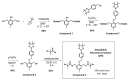
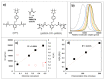
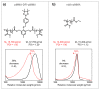
Mid-chain degradable polymers can be prepared by atom transfer radical polymerization from difunctional initiators that include triggers for the desired stimuli. While many difunctional initiators can respond to reducing conditions, procedures to prepare difunctional initiators that respond to oxidizing conditions are significantly less available in the literature. Here, a difunctional initiator incorporating an oxidizable boronic ester trigger was synthesized over four steps using simple and scalable procedures. Methyl methacrylate was polymerized by atom transfer radical polymerization using this initiator, and the polymerization kinetics were consistent with a controlled polymerization. The polymer synthesized using the difunctional initiator was found to decrease in molecular weight by 58% in the presence of hydrogen peroxide, while a control experiment using poly(methyl methacrylate) without a degradable linkage showed a much smaller decrease in molecular weight of only 9%. These observed molecular weight decreases were consistent with cleavage of the difunctional initiator via a quinone methide shift and hydrolysis of the methyl ester pendent groups in both polymers, and both polymers increased in polydispersity after oxidative degradation.
Keywords: ATRP; boronic ester; degradable polymer; difunctional initiator.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


Similar articles
-
Degradable-brushed pHEMA-pDMAEMA synthesized via ATRP and click chemistry for gene delivery.Bioconjug Chem. 2007 Nov-Dec;18(6):2077-84. doi: 10.1021/bc0701186. Epub 2007 Oct 10. Bioconjug Chem. 2007. PMID: 17927133
-
Comparative Study of Polymer-Grafted BaTiO3 Nanoparticles Synthesized Using Normal ATRP as Well as ATRP and ARGET-ATRP with Sacrificial Initiator with a Focus on Controlling the Polymer Graft Density and Molecular Weight.Molecules. 2023 May 30;28(11):4444. doi: 10.3390/molecules28114444. Molecules. 2023. PMID: 37298920 Free PMC article.
-
Designed amino acid ATRP initiators for the synthesis of biohybrid materials.J Am Chem Soc. 2008 Jan 23;130(3):1041-7. doi: 10.1021/ja0772546. J Am Chem Soc. 2008. PMID: 18161975
-
Development of new atom transfer radical polymerization system by iron (III)-metal salts without using any external initiator and reducing agent.Macromol Rapid Commun. 2013 Aug;34(15):1225-30. doi: 10.1002/marc.201300221. Epub 2013 May 2. Macromol Rapid Commun. 2013. PMID: 23637082
-
Diffusion-regulated phase-transfer catalysis for atom transfer radical polymerization of methyl methacrylate in an aqueous/organic biphasic system.Macromol Rapid Commun. 2015 Mar;36(6):538-46. doi: 10.1002/marc.201400639. Epub 2015 Feb 3. Macromol Rapid Commun. 2015. PMID: 25648231
Cited by
-
Synthesis and Characterization of ABA-Type Triblock Copolymers Using Novel Bifunctional PS, PMMA, and PCL Macroinitiators Bearing p-xylene-bis(2-mercaptoethyloxy) Core.Polymers (Basel). 2023 Sep 18;15(18):3813. doi: 10.3390/polym15183813. Polymers (Basel). 2023. PMID: 37765667 Free PMC article.
References
-
- Kato M., Kamigaito M., Sawamoto M., Higashimura T. Polymerization of Methyl Methacrylate with the Carbon Tetrachloride/Dichlorotris-(triphenylphosphine)ruthenium(II)/Methylaluminum Bis(2,6-di-tert-butylphenoxide) Initiating System: Possibility of Living Radical Polymerization. Macromolecules. 1995;28:1721–1723. doi: 10.1021/ma00109a056. - DOI
-
- Wang J.-S., Matyjaszewski K. Controlled/“Living” Radical Polymerization. Halogen Atom Transfer Radical Polymerization Promoted by a Cu(I)/Cu(II) Redox Process. Macromolecules. 1995;28:7901. doi: 10.1021/ma00127a042. - DOI
-
- Wang J.-S., Matyjaszewski K. “Living”/Controlled Radical Polymerization. Transition-Metal-Catalyzed Atom Transfer Radical Polymerization in the Presence of a Conventional Radical Initiator. Macromolecules. 1995;28:7572. doi: 10.1021/ma00126a041. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

