Synthesis and In Vitro Characterization of Ascorbyl Palmitate-Loaded Solid Lipid Nanoparticles
- PMID: 35566920
- PMCID: PMC9102913
- DOI: 10.3390/polym14091751
Synthesis and In Vitro Characterization of Ascorbyl Palmitate-Loaded Solid Lipid Nanoparticles
Abstract
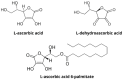
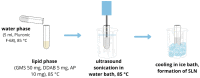
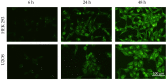
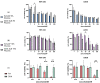
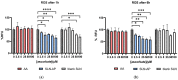
Antitumor applications of ascorbic acid (AA) and its oxidized form dehydroascorbic acid (DHA) can be quite challenging due to their instability and sensitivity to degradation in aqueous media. To overcome this obstacle, we have synthesized solid lipid nanoparticles loaded with ascorbyl palmitate (SLN-AP) with variations in proportions of the polymer Pluronic F-68. SLNs were synthesized using the hot homogenization method, characterized by measuring the particle size, polydispersity, zeta potential and visualized by TEM. To investigate the cellular uptake of the SLN, we have incorporated coumarin-6 into the same SLN formulation and followed their successful uptake for 48 h. We have tested the cytotoxicity of the SLN formulations and free ascorbate forms, AA and DHA, on HEK 293 and U2OS cell lines by MTT assay. The SLN-AP in both formulations have a cytotoxic effect at lower concentrations when compared to ascorbate applied the form of AA or DHA. Better selectivity for targeting tumor cell line was observed with 3% Pluronic F-68. The antioxidative effect of the SLN-AP was observed as early as 1 h after the treatment with a small dose of ascorbate applied (5 µM). SLN-AP formulation with 3% Pluronic F-68 needs to be further optimized as an ascorbate carrier due to its intrinsic cytotoxicity.
Keywords: antitumor effect; ascorbate; ascorbyl palmitate; cellular uptake; drug delivery; nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for application of ascorbyl palmitate.Pharmazie. 2005 Aug;60(8):577-82. Pharmazie. 2005. PMID: 16124399
-
Self-reporter shikonin-Act-loaded solid lipid nanoparticle: formulation, physicochemical characterization and geno/cytotoxicity evaluation.Eur J Pharm Sci. 2014 Aug 1;59:49-57. doi: 10.1016/j.ejps.2014.04.009. Epub 2014 Apr 23. Eur J Pharm Sci. 2014. PMID: 24768857
-
Formulation and in vitro characterization of domperidone loaded solid lipid nanoparticles and nanostructured lipid carriers.Daru. 2011;19(1):23-32. Daru. 2011. PMID: 22615636 Free PMC article.
-
Ascorbyl palmitate-incorporated paclitaxel-loaded composite nanoparticles for synergistic anti-tumoral therapy.Drug Deliv. 2017 Nov;24(1):1230-1242. doi: 10.1080/10717544.2017.1370619. Drug Deliv. 2017. PMID: 28856937 Free PMC article.
-
Final report of the safety assessment of L-Ascorbic Acid, Calcium Ascorbate, Magnesium Ascorbate, Magnesium Ascorbyl Phosphate, Sodium Ascorbate, and Sodium Ascorbyl Phosphate as used in cosmetics.Int J Toxicol. 2005;24 Suppl 2:51-111. doi: 10.1080/10915810590953851. Int J Toxicol. 2005. PMID: 16154915 Review.
Cited by
-
Solid Lipid Nanoparticles: Review of the Current Research on Encapsulation and Delivery Systems for Active and Antioxidant Compounds.Antioxidants (Basel). 2023 Mar 3;12(3):633. doi: 10.3390/antiox12030633. Antioxidants (Basel). 2023. PMID: 36978881 Free PMC article. Review.
-
The Biochemistry and Effectiveness of Antioxidants in Food, Fruits, and Marine Algae.Antioxidants (Basel). 2023 Apr 2;12(4):860. doi: 10.3390/antiox12040860. Antioxidants (Basel). 2023. PMID: 37107235 Free PMC article. Review.
-
Solid Lipid Nanoparticles Based on Monosubstituted Pillar[5]arenes: Chemoselective Synthesis of Macrocycles and Their Supramolecular Self-Assembly.Nanomaterials (Basel). 2022 Nov 30;12(23):4266. doi: 10.3390/nano12234266. Nanomaterials (Basel). 2022. PMID: 36500889 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

