Hydrodynamic Characteristics and Conformational Parameters of Ferrocene-Terpyridine-Based Polymers
- PMID: 35566943
- PMCID: PMC9104623
- DOI: 10.3390/polym14091776
Hydrodynamic Characteristics and Conformational Parameters of Ferrocene-Terpyridine-Based Polymers
Abstract
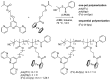
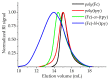
Nowadays, the study of metallopolymers is one of the fastest growing areas of polymer science. Metallopolymers have great potential for application in multiple technological and various biomedical processes. The macromolecules with the possibility of varying the number and type of metal ions along the entire length of the polymer chain are of particular interest. In this regard, this study presents results on two successfully synthesized homopolymers, random and block copolymers based on PMMA, containing ferrocene and terpyridine moieties in the side chain. Different architectures of copolymers may attribute interesting properties when creating complexes with various metal ions. A detailed hydrodynamic study of these structures was carried out, the consistency of hydrodynamic data was established using the concept of a hydrodynamic invariant, the absolute values of the molar masses of the studied objects were calculated, and the conformational parameters of macromolecules were determined. Using the Fixman-Stockmayer theory, the equilibrium rigidities of the studied systems were calculated and the relationship between the chemical structure and conformational characteristics was established. The studied copolymers can be attributed to the class of flexible-chain macromolecules. An increase in the equilibrium rigidity value with an increase of the side chain, which is characteristic of comb-shaped polymers, was determined.
Keywords: analytical ultracentrifugation; complexation; equilibrium rigidity; light scattering; metallopolymers; polymer chain conformation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Metallo-Supramolecular Complexation Behavior of Terpyridine- and Ferrocene-Based Polymers in Solution-A Molecular Hydrodynamics Perspective.Polymers (Basel). 2022 Feb 26;14(5):944. doi: 10.3390/polym14050944. Polymers (Basel). 2022. PMID: 35267767 Free PMC article.
-
Lanthanoids Goes Healing: Lanthanoidic Metallopolymers and Their Scratch Closure Behavior.Polymers (Basel). 2020 Apr 6;12(4):838. doi: 10.3390/polym12040838. Polymers (Basel). 2020. PMID: 32268577 Free PMC article.
-
Hydrodynamic Analysis Resolves the Pharmaceutically-Relevant Absolute Molar Mass and Solution Properties of Synthetic Poly(ethylene glycol)s Created by Varying Initiation Sites.Anal Chem. 2017 Jan 17;89(2):1185-1193. doi: 10.1021/acs.analchem.6b03615. Epub 2016 Dec 22. Anal Chem. 2017. PMID: 27936605
-
Recent Trends in Metallopolymer Design: Redox-Controlled Surfaces, Porous Membranes, and Switchable Optical Materials Using Ferrocene-Containing Polymers.Chemistry. 2018 Jul 17;24(40):10006-10021. doi: 10.1002/chem.201800412. Epub 2018 May 4. Chemistry. 2018. PMID: 29532972 Review.
-
Secondary Structure in Nonpeptidic Supramolecular Block Copolymers.Acc Chem Res. 2021 May 18;54(10):2397-2408. doi: 10.1021/acs.accounts.1c00028. Epub 2021 Apr 29. Acc Chem Res. 2021. PMID: 33914498 Review.
Cited by
-
Self-Healing of Polymers and Polymer Composites.Polymers (Basel). 2022 Dec 9;14(24):5404. doi: 10.3390/polym14245404. Polymers (Basel). 2022. PMID: 36559772 Free PMC article. Review.
References
-
- Durkee D.A., Eitouni H.B., Gomez E.D., Ellsworth M.W., Bell A.T., Balsara N.P. Catalysts from Self-Assembled Organometallic Block Copolymers. Adv. Mater. 2005;17:2003–2006. doi: 10.1002/adma.200500352. - DOI
-
- Hardy C.G., Zhang J., Yan Y., Ren L., Tang C. Metallopolymers with transition metals in the side-chain by living and controlled polymerization techniques. Prog. Polym. Sci. 2014;39:1742–1796. doi: 10.1016/j.progpolymsci.2014.03.002. - DOI
-
- Holliday B.J., Stanford T.B., Swager T.M. Chemoresistive Gas-Phase Nitric Oxide Sensing with Cobalt-Containing Conducting Metallopolymers. Chem. Mater. 2006;18:5649–5651. doi: 10.1021/cm0620077. - DOI
-
- Ho C.-L., Wong W.-Y. Metal-containing polymers: Facile tuning of photophysical traits and emerging applications in organic electronics and photonics. Coord. Chem. Rev. 2011;255:2469–2502. doi: 10.1016/j.ccr.2011.01.052. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

