Biogenic Synthesis and Characterization of Chitosan-CuO Nanocomposite and Evaluation of Antibacterial Activity against Gram-Positive and -Negative Bacteria
- PMID: 35567006
- PMCID: PMC9104765
- DOI: 10.3390/polym14091832
Biogenic Synthesis and Characterization of Chitosan-CuO Nanocomposite and Evaluation of Antibacterial Activity against Gram-Positive and -Negative Bacteria
Abstract
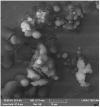
Chitosan-copper oxide (CHT-CuO) nanocomposite was synthesized using olive leaf extract (OLE) as reducing agent and CuSO4⋅5H2O as precursor. CHT-CuO nanocomposite was prepared using an in situ method in which OLE was added to a solution of chitosan and CuSO4⋅5H2O mixture in the ratio of 1:5 (v/v) and heated at a temperature of 90 °C. The obtained CHT-CuO nanocomposite was characterized using field emission scanning electron microscopy (FE-SEM), X-ray diffraction (XRD), ultraviolet-visible (UV-Vis) spectrophotometry, energy-dispersive X-ray spectroscopy (EDAX), Fourier transform infrared spectroscopy (FTIR), and high-resolution transmission electron microscopy (TEM). TEM results indicated that CHT-CuO nanocomposite are spherical in shape with size ranging from 3.5 to 6.0 nm. Antibacterial activity of the synthesized nanocomposites was evaluated against Gram-positive (Bacillus cereus, Staphyloccous haemolytica and Micrococcus Luteus) and Gram-negative (Escherichia coli, Pseudomonas citronellolis, Pseudomonas aeruginosa, kliebisella sp., Bradyrhizobium japonicum and Ralstonia pickettii) species by cup platting or disc diffusion method. Overall, against all tested bacterial strains, the diameters of the inhibition zone of the three nanocomposites fell between 6 and 24 mm, and the order of the antimicrobial activity was as follows: CuO-1.0 > CuO-0.5 > CuO-2.0. The reference antibiotic amoxicillin and ciprofloxacin showed greater activity based on the diameter of zones of inhibition (between 15−32 mm) except for S. heamolytica and P. citronellolis bacteria strains. The nanocomposites MIC/MBC were between 0.1 and 0.01% against all tested bacteria, except S. heamolityca (>0.1%). Based on MIC/MBC values, CuO-0.5 and CuO-1.0 were more active than CuO-2.0, in line with the observations from the disc diffusion experiment. The findings indicate that these nanocomposites are efficacious against bacteria; however, Gram-positive bacteria were less susceptible. The synthesized CHT-CuO nanocomposite shows promising antimicrobial activities and could be utilized as an antibacterial agent in packaging and medical applications.
Keywords: antibacterial activity; chitosan; copper oxide; nanocomposite; olive leaf extract.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Das B., Moumita S., Ghosh S., Khan M.I., Indira D., Jayabalan R., Tripathy S.K., Mishra A., Balasubramanian P. Biosynthesis of magnesium oxide (MgO) nanoflakes by using leaf extract of Bauhinia purpurea and evaluation of its antibacterial property against Staphylococcus aureus. Mater. Sci. Eng. C. 2018;91:436–444. doi: 10.1016/j.msec.2018.05.059. - DOI - PubMed
LinkOut - more resources
Full Text Sources

