Aquaponics-Derived Tilapia Skin Collagen for Biomaterials Development
- PMID: 35567034
- PMCID: PMC9103308
- DOI: 10.3390/polym14091865
Aquaponics-Derived Tilapia Skin Collagen for Biomaterials Development
Abstract
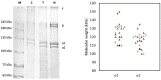
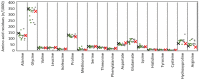
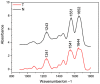
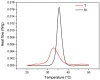
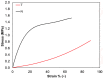
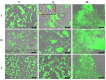
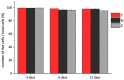
Collagen is one of the most widely used biomaterials in health-related sectors. The industrial production of collagen mostly relies on its extraction from mammals, but several issues limited its use. In the last two decades, marine organisms attracted interest as safe, abundant, and alternative source for collagen extraction. In particular, the possibility to valorize the huge quantity of fish industry waste and byproducts as collagen source reinforced perception of fish collagen as eco-friendlier and particularly attractive in terms of profitability and cost-effectiveness. Especially fish byproducts from eco-sustainable aquaponics production allow for fish biomass with additional added value and controlled properties over time. Among fish species, Oreochromis niloticus is one of the most widely bred fish in large-scale aquaculture and aquaponics systems. In this work, type I collagen was extracted from aquaponics-raised Tilapia skin and characterized from a chemical, physical, mechanical, and biological point of view in comparison with a commercially available analog. Performed analysis confirmed that the proprietary process optimized for type I collagen extraction allowed to isolate pure native collagen and to preserve its native conformational structure. Preliminary cellular studies performed with mouse fibroblasts indicated its optimal biocompatibility. All data confirmed the eligibility of the extracted Tilapia-derived native type I collagen as a biomaterial for healthcare applications.
Keywords: aquaponic; biomaterials; skin; tilapia; type I collagen.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Salvatore L., Gallo N., Natali M.L., Terzi A., Sannino A., Madaghiele M. Mimicking the Hierarchical Organization of Natural Collagen: Toward the Development of Ideal Scaffolding Material for Tissue Regeneration. Front. Bioeng. Biotechnol. 2021;9:644595. doi: 10.3389/fbioe.2021.644595. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

