Recent Advances in Fluorescence Recovery after Photobleaching for Decoupling Transport and Kinetics of Biomacromolecules in Cellular Physiology
- PMID: 35567083
- PMCID: PMC9105003
- DOI: 10.3390/polym14091913
Recent Advances in Fluorescence Recovery after Photobleaching for Decoupling Transport and Kinetics of Biomacromolecules in Cellular Physiology
Abstract
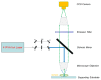
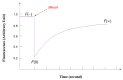
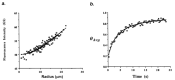
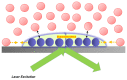
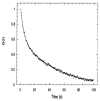
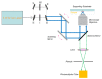
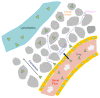
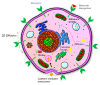
Among the new molecular tools available to scientists and engineers, some of the most useful include fluorescently tagged biomolecules. Tools, such as green fluorescence protein (GFP), have been applied to perform semi-quantitative studies on biological signal transduction and cellular structural dynamics involved in the physiology of healthy and disease states. Such studies focus on drug pharmacokinetics, receptor-mediated endocytosis, nuclear mechanobiology, viral infections, and cancer metastasis. In 1976, fluorescence recovery after photobleaching (FRAP), which involves the monitoring of fluorescence emission recovery within a photobleached spot, was developed. FRAP allowed investigators to probe two-dimensional (2D) diffusion of fluorescently-labelled biomolecules. Since then, FRAP has been refined through the advancements of optics, charged-coupled-device (CCD) cameras, confocal microscopes, and molecular probes. FRAP is now a highly quantitative tool used for transport and kinetic studies in the cytosol, organelles, and membrane of a cell. In this work, the authors intend to provide a review of recent advances in FRAP. The authors include epifluorescence spot FRAP, total internal reflection (TIR)/FRAP, and confocal microscope-based FRAP. The underlying mathematical models are also described. Finally, our understanding of coupled transport and kinetics as determined by FRAP will be discussed and the potential for future advances suggested.
Keywords: bio-interfaces; biomolecules; biophysical techniques; fluorescence recovery after photobleaching; polymers; reaction; transport.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

