Role of Nodulation-Enhancing Rhizobacteria in the Promotion of Medicago sativa Development in Nutrient-Poor Soils
- PMID: 35567168
- PMCID: PMC9099972
- DOI: 10.3390/plants11091164
Role of Nodulation-Enhancing Rhizobacteria in the Promotion of Medicago sativa Development in Nutrient-Poor Soils
Abstract
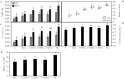
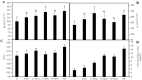
Legumes are usually used as cover crops to improve soil quality due to the biological nitrogen fixation that occurs due to the interaction of legumes and rhizobia. This symbiosis can be used to recover degraded soils using legumes as pioneer plants. In this work, we screened for bacteria that improve the legume-rhizobia interaction in nutrient-poor soils. Fourteen phosphate solubilizer-strains were isolated, showing at least three out of the five tested plant growth promoting properties. Furthermore, cellulase, protease, pectinase, and chitinase activities were detected in three of the isolated strains. Pseudomonas sp. L1, Chryseobacterium soli L2, and Priestia megaterium L3 were selected to inoculate seeds and plants of Medicago sativa using a nutrient-poor soil as substrate under greenhouse conditions. The effects of the three bacteria individually and in consortium showed more vigorous plants with increased numbers of nodules and a higher nitrogen content than non-inoculated plants. Moreover, bacterial inoculation increased plants' antioxidant activities and improved their development in nutrient-poor soils, suggesting an important role in the stress mechanisms of plants. In conclusion, the selected strains are nodulation-enhancing rhizobacteria that improve leguminous plants growth and nodulation in nutrient-poor soils and could be used by sustainable agriculture to promote plants' development in degraded soils.
Keywords: Medicago; abiotic stress; biofertilizers; degraded soils; legumes; nodulation-enhancing rhizobacteria (NER); nutrient poverty; plant growth-promoting rhizobacteria (PGPR).
Conflict of interest statement
The authors declare that they have no known competing financial or personal interest that could have appeared to influence the work reported in this paper.
Figures



References
-
- Azani N., Babineau M., Bailey C.D., Banks H., Barbosa A.R., Pinto R.B., Boatwright J.S., Borges L.M., Brown G.K., Bruneau A., et al. A new subfamily classification of the leguminosae based on a taxonomically comprehensive phylogeny: The Legume Phylogeny Working Group (LPWG) Taxon. 2017;66:44–77. doi: 10.12705/661.3. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

