Morphological and Molecular Characterization of Some Alternaria Species Isolated from Tomato Fruits Concerning Mycotoxin Production and Polyketide Synthase Genes
- PMID: 35567169
- PMCID: PMC9103205
- DOI: 10.3390/plants11091168
Morphological and Molecular Characterization of Some Alternaria Species Isolated from Tomato Fruits Concerning Mycotoxin Production and Polyketide Synthase Genes
Abstract
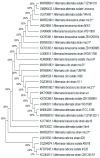
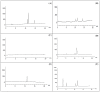
Tomatoes (Lycopersicon esculentum) are one of the main crops grown in Egypt. The fungal black spot illness of fruits is usually associated with the secretion of mycotoxin by Alternaria toxigenic species. Twenty Alternaria isolates were isolated from infected tomatoes fruits by baiting technique, morphologically identified to species level, and confirmed using Internal Transcribed Spacer (ITS) gene sequencing. ITS gene sequencing of fragments obtained 547, 547, 542, 554, and 547 bp for A. alternata, A. brassicicola, A. citri, A. radicina, and A. tenuissima, respectively. Alternaria species were investigated for mycotoxin production using the high-performance liquid chromatography (HPLC) technique. The data from the HPLC analysis showed that the mycotoxins were determined in four out of five Alternaria species, with the incidence ranging from 0.89-9.85 µg/mL of fungal extract at different retention times. Alternaria alternata was the most active species and produced three types of toxins. Polyketide synthase genes (pksH and pksJ) which are involved in the Alternaria toxin's biosynthesis were also amplified from the DNA of Alternaria species.
Keywords: Alternaria; HPLC; internal transcribed spacer; mycotoxins; pksH & pksJ genes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Ostry V. Alternaria mycotoxins: An overview of chemical characterization, producers, toxicity, analysis and occurrence in foodstuffs. World Mycotoxin J. 2008;1:175–188. doi: 10.3920/WMJ2008.x013. - DOI
-
- Pinto V.E.F., Patriarca A. Alternaria species, and their associated mycotoxins. In: Moretti A., Susca A., editors. Mycotoxigenic Fungi: Methods in Molecular Biology. Volume 1542. Humana Press; New York, NY, USA: 2017. pp. 13–32. Chapter 2. - PubMed
-
- Hussein M.A., Voigt K. Phylogenetic and enzymatic variability of Alternaria species isolated from various substrates in Qena governorate of Upper Egypt. Arch. Phytopathol. Plant Prot. 2019;52:530–541. doi: 10.1080/03235408.2019.1651580. - DOI
LinkOut - more resources
Full Text Sources

