Advances in Understanding the Genetic Basis of Fatty Acids Biosynthesis in Perilla: An Update
- PMID: 35567213
- PMCID: PMC9099743
- DOI: 10.3390/plants11091207
Advances in Understanding the Genetic Basis of Fatty Acids Biosynthesis in Perilla: An Update
Abstract
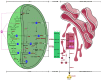
Perilla, also termed as purple mint, Chinese basil, or Perilla mint, is a flavoring herb widely used in East Asia. Both crude oil and essential oil are employed for consumption as well as industrial purposes. Fatty acids (FAs) biosynthesis and oil body assemblies in Perilla have been extensively investigated over the last three decades. Recent advances have been made in order to reveal the enzymes involved in the fatty acid biosynthesis in Perilla. Among those fatty acids, alpha-linolenic acid retained the attention of scientists mainly due to its medicinal and nutraceutical properties. Lipids synthesis in Perilla exhibited similarities with Arabidopsis thaliana lipids' pathway. The homologous coding genes for polyunsaturated fatty acid desaturases, transcription factors, and major acyl-related enzymes have been found in Perilla via de novo transcriptome profiling, genome-wide association study, and in silico whole-genome screening. The identified genes covered de novo fatty acid synthesis, acyl-CoA dependent Kennedy pathway, acyl-CoA independent pathway, Triacylglycerols (TAGs) assembly, and acyl editing of phosphatidylcholine. In addition to the enzymes, transcription factors including WRINKLED, FUSCA3, LEAFY COTYLEDON1, and ABSCISIC ACID INSENSITIVE3 have been suggested. Meanwhile, the epigenome aspect impacting the transcriptional regulation of FAs is still unclear and might require more attention from the scientific community. This review mainly outlines the identification of the key gene master players involved in Perilla FAs biosynthesis and TAGs assembly that have been identified in recent years. With the recent advances in genomics resources regarding this orphan crop, we provided an updated overview of the recent contributions into the comprehension of the genetic background of fatty acid biosynthesis. The provided resources can be useful for further usage in oil-bioengineering and the design of alpha-linolenic acid-boosted Perilla genotypes in the future.
Keywords: Perilla; fatty acid biosynthesis; fatty acid desaturase; genomics; oil crop; transcription factor; transcriptomics; triacylglycerol biosynthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Improving the Traits of Perilla frutescens (L.) Britt Using Gene Editing Technology.Plants (Basel). 2024 May 25;13(11):1466. doi: 10.3390/plants13111466. Plants (Basel). 2024. PMID: 38891275 Free PMC article. Review.
-
RNA Sequencing and Coexpression Analysis Reveal Key Genes Involved in α-Linolenic Acid Biosynthesis in Perilla frutescens Seed.Int J Mol Sci. 2017 Nov 16;18(11):2433. doi: 10.3390/ijms18112433. Int J Mol Sci. 2017. PMID: 29144390 Free PMC article.
-
Transcriptome analysis and identification of genes associated with ω-3 fatty acid biosynthesis in Perilla frutescens (L.) var. frutescens.BMC Genomics. 2016 Jun 24;17:474. doi: 10.1186/s12864-016-2805-0. BMC Genomics. 2016. PMID: 27342315 Free PMC article.
-
Genome-Wide Analysis of the Fatty Acid Desaturase Gene Family Reveals the Key Role of PfFAD3 in α-Linolenic Acid Biosynthesis in Perilla Seeds.Front Genet. 2021 Nov 24;12:735862. doi: 10.3389/fgene.2021.735862. eCollection 2021. Front Genet. 2021. PMID: 34899834 Free PMC article.
-
A comprehensive review on nutritional, nutraceutical, and industrial perspectives of perilla (Perilla frutscens L.) seeds - An orphan oilseed crop.Heliyon. 2024 Jun 18;10(12):e33281. doi: 10.1016/j.heliyon.2024.e33281. eCollection 2024 Jun 30. Heliyon. 2024. PMID: 39022021 Free PMC article. Review.
Cited by
-
Improving the Traits of Perilla frutescens (L.) Britt Using Gene Editing Technology.Plants (Basel). 2024 May 25;13(11):1466. doi: 10.3390/plants13111466. Plants (Basel). 2024. PMID: 38891275 Free PMC article. Review.
-
Perilla frutescens L.: a dynamic food crop worthy of future challenges.Front Nutr. 2023 Jun 1;10:1130927. doi: 10.3389/fnut.2023.1130927. eCollection 2023. Front Nutr. 2023. PMID: 37324746 Free PMC article. No abstract available.
-
Proteomics reveals the significance of vacuole Pi transporter in the adaptability of Brassica napus to Pi deprivation.Front Plant Sci. 2024 Mar 25;15:1340867. doi: 10.3389/fpls.2024.1340867. eCollection 2024. Front Plant Sci. 2024. PMID: 38590751 Free PMC article.
-
Key FAD2, FAD3, and SAD Genes Involved in the Fatty Acid Synthesis in Flax Identified Based on Genomic and Transcriptomic Data.Int J Mol Sci. 2023 Oct 4;24(19):14885. doi: 10.3390/ijms241914885. Int J Mol Sci. 2023. PMID: 37834335 Free PMC article.
-
The Genome of the Korean Island-Originated Perilla citriodora 'Jeju17' Sheds Light on Its Environmental Adaptation and Fatty Acid and Lipid Production Pathways.Genes (Basel). 2023 Sep 30;14(10):1898. doi: 10.3390/genes14101898. Genes (Basel). 2023. PMID: 37895247 Free PMC article.
References
-
- Nitta M., Lee J.K., Kang C.W., Katsuta M., Yasumoto S., Liu D., Nagamine T., Ohnishi O. The Distribution of Perilla Species. Genet. Resour. Crop Evol. 2005;52:797–804. doi: 10.1007/s10722-003-6017-5. - DOI
-
- Banno N., Akihisa T., Tokuda H., Yasukawa K., Higashihara H., Ukiya M., Watanabe K., Kimura Y., Hasegawa J.I., Nishino H. Triterpene acids from the leaves of Perilla frutescens and their anti-inflammatory and antitumor-promoting effects. Biosci. Biotechnol. Biochem. 2004;68:85–90. doi: 10.1271/bbb.68.85. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

