Bryophyte Spores Tolerate High Desiccation Levels and Exposure to Cryogenic Temperatures but Contain Storage Lipids and Chlorophyll: Understanding the Essential Traits Needed for the Creation of Bryophyte Spore Banks
- PMID: 35567263
- PMCID: PMC9100633
- DOI: 10.3390/plants11091262
Bryophyte Spores Tolerate High Desiccation Levels and Exposure to Cryogenic Temperatures but Contain Storage Lipids and Chlorophyll: Understanding the Essential Traits Needed for the Creation of Bryophyte Spore Banks
Abstract
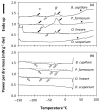
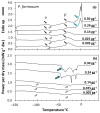
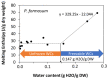
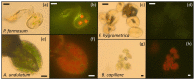
Understanding the desiccation and freezing tolerance of bryophyte spores is vital to explain how plants conquered land and current species distribution patterns and help to develop efficient ex situ conservation methods. However, knowledge of these traits is scarce. We investigated tolerance to drying (at 15% relative humidity [RH] for two weeks) and freezing (1 h exposure to liquid nitrogen) on the spores of 12 bryophyte species (23 accessions) from the UK. The presence of storage lipids and their thermal fingerprint, and the levels of unfrozen water content, were determined by differential scanning calorimetry (DSC). The presence of chlorophyll in dry spores was detected by fluorescence microscopy. All species and accessions tested tolerated the drying and freezing levels studied. DSC suggested that 4.1−29.3% of the dry mass is storage lipids, with crystallization and melting temperatures peaking at around −30 °C. Unfrozen water content was determined <0.147 g H2O g−1 dry weight (DW). Most of the spores investigated showed the presence of chlorophyll in the cytoplasm by red autofluorescence. Bryophyte spores can be stored dry at low temperatures, such as orthodox seeds, supporting the creation of bryophyte spore banks. However, the presence of storage lipids and chlorophyll in the cytoplasm may reduce spore longevity during conventional storage at −20 °C. Alternatively, cryogenic spore storage is possible.
Keywords: chlorophyll; cryopreservation; desiccation tolerance; differential scanning calorimetry; ex situ conservation; in vitro germination; lipid crystallization; liquid nitrogen; unfrozen water content.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Effects of temperature and desiccation on ex situ conservation of nongreen fern spores.Am J Bot. 2012 Apr;99(4):721-9. doi: 10.3732/ajb.1100257. Epub 2012 Mar 20. Am J Bot. 2012. PMID: 22434770
-
Calorimetric properties of water and triacylglycerols in fern spores relating to storage at cryogenic temperatures.Cryobiology. 2007 Aug;55(1):1-9. doi: 10.1016/j.cryobiol.2007.03.006. Epub 2007 Apr 14. Cryobiology. 2007. PMID: 17553480
-
Cryopreservation of Seeds and Seed Embryos in Orthodox-, Intermediate-, and Recalcitrant-Seeded Species.Methods Mol Biol. 2021;2180:663-682. doi: 10.1007/978-1-0716-0783-1_36. Methods Mol Biol. 2021. PMID: 32797442
-
Choosing the Right Path for the Successful Storage of Seeds.Plants (Basel). 2022 Dec 23;12(1):72. doi: 10.3390/plants12010072. Plants (Basel). 2022. PMID: 36616200 Free PMC article. Review.
-
The Orthodox Dry Seeds Are Alive: A Clear Example of Desiccation Tolerance.Plants (Basel). 2021 Dec 22;11(1):20. doi: 10.3390/plants11010020. Plants (Basel). 2021. PMID: 35009023 Free PMC article. Review.
Cited by
-
Dynamic Membrane Lipid Changes in Physcomitrium patens Reveal Developmental and Environmental Adaptations.Biology (Basel). 2024 Sep 16;13(9):726. doi: 10.3390/biology13090726. Biology (Basel). 2024. PMID: 39336153 Free PMC article.
References
-
- Graham L., Lewis L.A., Taylor W., Wellman C., Cook M. Early Terrestrialization: Transition from Algal to bryophyte Grade. In: Hanson D., Rice S., editors. Photosynthesis in Bryophytes and Early Land Plants; Advances in Photosynthesis and Respiration (Including Bioenergy and Related Processes) Volume 37. Springer; Dordrecht, The Netherlands: 2014. pp. 9–28.
-
- van Zanten B.O., Pòcs T. Distribution and dispersal of bryophytes. Adv. Bryol. 1981;1:479–562.
-
- Page C.N. Ecological strategies in fern evolution: A neopteridological overview. Rev. Palaeobot. Palynol. 2002;119:1–33. doi: 10.1016/S0034-6667(01)00127-0. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

