T-cell recovery and evidence of persistent immune activation 12 months after severe COVID-19
- PMID: 35567391
- PMCID: PMC9347640
- DOI: 10.1111/all.15372
T-cell recovery and evidence of persistent immune activation 12 months after severe COVID-19
Abstract
Background: T-cell lymphopenia and functional impairment is a hallmark of severe acute coronavirus disease 2019 (COVID-19). How T-cell numbers and function evolve at later timepoints after clinical recovery remains poorly investigated.
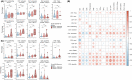
Methods: We prospectively enrolled and longitudinally sampled 173 individuals with asymptomatic to critical COVID-19 and analyzed phenotypic and functional characteristics of T cells using flow cytometry, 40-parameter mass cytometry, targeted proteomics, and functional assays.
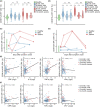
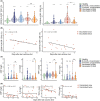
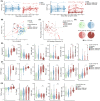
Results: The extensive T-cell lymphopenia observed particularly in patients with severe COVID-19 during acute infection had recovered 6 months after infection, which was accompanied by a normalization of functional T-cell responses to common viral antigens. We detected persisting CD4+ and CD8+ T-cell activation up to 12 months after infection, in patients with mild and severe COVID-19, as measured by increased HLA-DR and CD38 expression on these cells. Persistent T-cell activation after COVID-19 was independent of administration of a COVID-19 vaccine post-infection. Furthermore, we identified a subgroup of patients with severe COVID-19 that presented with persistently low CD8+ T-cell counts at follow-up and exhibited a distinct phenotype during acute infection consisting of a dysfunctional T-cell response and signs of excessive pro-inflammatory cytokine production.
Conclusion: Our study suggests that T-cell numbers and function recover in most patients after COVID-19. However, we find evidence of persistent T-cell activation up to 12 months after infection and describe a subgroup of severe COVID-19 patients with persistently low CD8+ T-cell counts exhibiting a dysregulated immune response during acute infection.
Keywords: COVID-19; SARS-CoV-2; T cells; follow-up; recovery.
© 2022 The Authors. Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
Dr. Taeschler, Dr. Chevrier, Ms. Hasler, Dr. Baechli, Dr. Rudiger, Dr. Stüssi‐Helbling, Dr. Huber, and Dr. Deng have nothing to disclose. Dr. Adamo reports grants from Swiss Academy of Medical Sciences and University of Zurich, during the conduct of the study. Dr. Cervia reports grants from Swiss Academy of Medical Sciences, during the conduct of the study. Mr. Zurbuchen reports grants from Swiss Academy of Medical Sciences, during the conduct of the study. Dr. Raeber reports a Young Talents in Clinical Research Project Grant by the Swiss Academy of Medical Sciences and the G. & J. Bangerter‐Rhyner Foundation, during the conduct of the study. Dr. Bodenmiller reports grants from Swiss National Science Foundation, grants from Pandemic Fund of the University of Zurich, during the conduct of the study. Dr. Boyman reports grants from Swiss National Science Foundation, grants from Clinical Research Priority Program of University of Zurich, and an Innovation grant of University Hospital Zurich, during the conduct of the study. Dr. Nilsson reports grants from Swiss National Science Foundation, during the conduct of the study.
Figures

References
-
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239‐1242. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

