Cracking the cryptic code in amyotrophic lateral sclerosis and frontotemporal dementia: Towards therapeutic targets and biomarkers
- PMID: 35567447
- PMCID: PMC9098226
- DOI: 10.1002/ctm2.818
Cracking the cryptic code in amyotrophic lateral sclerosis and frontotemporal dementia: Towards therapeutic targets and biomarkers
Abstract
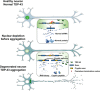
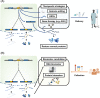
Amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) are two devastating human neurodegenerative diseases. A hallmark pathological feature of both diseases is the depletion of the RNA-binding protein TDP-43 from the nucleus in the brain and spinal cord of patients. A major function of TDP-43 is to repress the inclusion of cryptic exons during RNA splicing. When it becomes depleted from the nucleus in disease, this function is lost, and recently, several key cryptic splicing targets of TDP-43 have emerged, including STMN2, UNC13A, and others. UNC13A is a major ALS/FTD risk gene, and the genetic variations that increase the risk for disease seem to do so by making the gene more susceptible to cryptic exon inclusion when TDP-43 function is impaired. Here, we discuss the prospects and challenges of harnessing these cryptic splicing events as novel therapeutic targets and biomarkers. Deciphering this new cryptic code may be a touchstone for ALS and FTD diagnosis and treatment.
Keywords: ALS; FTD; TDP-43; UNC13A.
© 2022 The Authors. Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Conflict of interest statement
Aaron D. Gitler is a scientific founder of Maze Therapeutics.
Figures
References
-
- Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP‐43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130‐133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
