Interferon regulatory factor-5-dependent CD11c+ macrophages contribute to the formation of rupture-prone atherosclerotic plaques
- PMID: 35567557
- PMCID: PMC9113304
- DOI: 10.1093/eurheartj/ehab920
Interferon regulatory factor-5-dependent CD11c+ macrophages contribute to the formation of rupture-prone atherosclerotic plaques
Abstract
Aims: Inflammation is a key factor in atherosclerosis. The transcription factor interferon regulatory factor-5 (IRF5) drives macrophages towards a pro-inflammatory state. We investigated the role of IRF5 in human atherosclerosis and plaque stability.
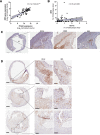
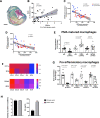
Methods and results: Bulk RNA sequencing from the Carotid Plaque Imaging Project biobank were used to mine associations between major macrophage associated genes and transcription factors and human symptomatic carotid disease. Immunohistochemistry, proximity extension assays, and Helios cytometry by time of flight (CyTOF) were used for validation. The effect of IRF5 deficiency on carotid plaque phenotype and rupture in ApoE-/- mice was studied in an inducible model of plaque rupture. Interferon regulatory factor-5 and ITGAX/CD11c were identified as the macrophage associated genes with the strongest associations with symptomatic carotid disease. Expression of IRF5 and ITGAX/CD11c correlated with the vulnerability index, pro-inflammatory plaque cytokine levels, necrotic core area, and with each other. Macrophages were the predominant CD11c-expressing immune cells in the plaque by CyTOF and immunohistochemistry. Interferon regulatory factor-5 immunopositive areas were predominantly found within CD11c+ areas with a predilection for the shoulder region, the area of the human plaque most prone to rupture. Accordingly, an inducible plaque rupture model of ApoE-/-Irf5-/- mice had significantly lower frequencies of carotid plaque ruptures, smaller necrotic cores, and less CD11c+ macrophages than their IRF5-competent counterparts.
Conclusion: Using complementary evidence from data from human carotid endarterectomies and a murine model of inducible rupture of carotid artery plaque in IRF5-deficient mice, we demonstrate a mechanistic link between the pro-inflammatory transcription factor IRF5, macrophage phenotype, plaque inflammation, and its vulnerability to rupture.
Keywords: Atherosclerosis; IRF5; Macrophages; Plaque rupture.
© The Author(s) 2022. Published by Oxford University Press on behalf of European Society of Cardiology.
Figures




Comment in
-
Advancing therapeutic targeting of the vulnerable plaque.Eur Heart J. 2022 May 14;43(19):1878-1880. doi: 10.1093/eurheartj/ehac060. Eur Heart J. 2022. PMID: 35567566 No abstract available.
References
-
- Burke AP, Farb A, Malcom GT, Liang Y-H, Smialek J, Virmani R. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med 1997;336:1276–1282. - PubMed
-
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005;352:1685–1695. - PubMed
-
- Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part I. Circulation 2003;108:1664–1672. - PubMed
-
- Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res 2014;114:1852–1866. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

