Thrombosis, cancer, and COVID-19
- PMID: 35567609
- PMCID: PMC9106567
- DOI: 10.1007/s00520-022-07098-z
Thrombosis, cancer, and COVID-19
Abstract
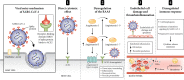
Cancer and coronavirus disease 2019 (COVID-19) have unusual similarities: they both result in a markedly elevated risk of thrombosis, exceptionally high D-dimer levels, and the failure of anticoagulation therapy in some cases. Cancer patients are more vulnerable to COVID-19 infection and have a higher mortality rate. Science has uncovered much about SARS-CoV-2, and made extraordinary and unprecedented progress on the development of various treatment strategies and COVID-19 vaccines. In this review, we discuss known data on cancer-associated thrombosis (CAT), SARS-CoV-2 infection, and COVID-19 vaccines and discuss considerations for managing CAT in patients with COVID-19. Cancer patients should be given priority for COVID-19 vaccination; however, they may demonstrate a weaker immune response to COVID-19 vaccines than the general population. Currently, the Centers for Disease Control and Prevention recommends an additional dose and booster shot of the COVID-19 vaccine after the primary series in patients undergoing active cancer treatment for solid tumors or hematological cancers, recipients of stem cell transplant within the last 2 years, those taking immunosuppressive medications, and those undergoing active treatment with high-dose corticosteroids or other drugs that suppress the immune response. The mainstay of thrombosis treatment in patients with cancer and COVID-19 is anticoagulation therapy.
Keywords: Anticoagulation; COVID-19; COVID-19 vaccines; Cancer; Thrombosis.
© 2022. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Clinical Characteristics and Pharmacological Management of COVID-19 Vaccine-Induced Immune Thrombotic Thrombocytopenia With Cerebral Venous Sinus Thrombosis: A Review.JAMA Cardiol. 2021 Dec 1;6(12):1451-1460. doi: 10.1001/jamacardio.2021.3444. JAMA Cardiol. 2021. PMID: 34374713 Review.
-
A randomized, double-blind, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of SARS-CoV-2 vaccine (inactivated, Vero cell): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Apr 13;22(1):276. doi: 10.1186/s13063-021-05180-1. Trials. 2021. PMID: 33849629 Free PMC article.
-
Vaccination Against SARS-CoV-2 in Lung Transplant Recipients: Immunogenicity, Efficacy and Safety.Front Immunol. 2022 Jun 1;13:906225. doi: 10.3389/fimmu.2022.906225. eCollection 2022. Front Immunol. 2022. PMID: 35720376 Free PMC article. Review.
-
A review of COVID-19-related thrombosis and anticoagulation strategies specific to the Asian population.Singapore Med J. 2022 Jul;63(7):350-361. doi: 10.11622/smedj.2020174. Epub 2020 Dec 2. Singapore Med J. 2022. PMID: 33264831 Free PMC article. Review.
-
Analysis of COVID-19 Incidence and Severity Among Adults Vaccinated With 2-Dose mRNA COVID-19 or Inactivated SARS-CoV-2 Vaccines With and Without Boosters in Singapore.JAMA Netw Open. 2022 Aug 1;5(8):e2228900. doi: 10.1001/jamanetworkopen.2022.28900. JAMA Netw Open. 2022. PMID: 36018588 Free PMC article.
Cited by
-
Analysis of the Risk Factors for Elevated D-Dimer Level After Breast Cancer Surgery: A Multicenter Study Based on Nursing Follow-Up Data.Front Oncol. 2022 Jul 19;12:772726. doi: 10.3389/fonc.2022.772726. eCollection 2022. Front Oncol. 2022. PMID: 35928882 Free PMC article.
-
Developing a novel SARS-CoV-2 risk index to predict the prognostic and therapeutic effects in acute myeloid leukemia.Heliyon. 2023 Nov 15;9(11):e22426. doi: 10.1016/j.heliyon.2023.e22426. eCollection 2023 Nov. Heliyon. 2023. PMID: 38074856 Free PMC article.
-
The signature of SARS-CoV-2-related genes predicts the immune therapeutic response and prognosis in breast cancer.BMC Med Genomics. 2024 Oct 31;17(1):260. doi: 10.1186/s12920-024-02032-0. BMC Med Genomics. 2024. PMID: 39482662 Free PMC article.
-
Proteomic and serologic assessments of responses to mRNA-1273 and BNT162b2 vaccines in human recipient sera.Front Immunol. 2025 Jan 27;15:1502458. doi: 10.3389/fimmu.2024.1502458. eCollection 2024. Front Immunol. 2025. PMID: 39931577 Free PMC article.
-
Inhibitory effect of napabucasin on arbidol metabolism and its mechanism research.Front Pharmacol. 2023 Nov 28;14:1292354. doi: 10.3389/fphar.2023.1292354. eCollection 2023. Front Pharmacol. 2023. PMID: 38094891 Free PMC article.
References
-
- Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19) 2021 [updated February 16, 2021. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-manageme... 20 January 2022
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

