Transcriptomic analysis of Stropharia rugosoannulata reveals carbohydrate metabolism and cold resistance mechanisms under low-temperature stress
- PMID: 35567721
- PMCID: PMC9107548
- DOI: 10.1186/s13568-022-01400-2
Transcriptomic analysis of Stropharia rugosoannulata reveals carbohydrate metabolism and cold resistance mechanisms under low-temperature stress
Erratum in
-
Correction: Transcriptomic analysis of Stropharia rugosoannulata reveals carbohydrate metabolism and cold resistance mechanisms under low-temperature stress.AMB Express. 2022 Jun 13;12(1):72. doi: 10.1186/s13568-022-01412-y. AMB Express. 2022. PMID: 35697966 Free PMC article. No abstract available.
Abstract
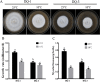
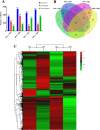
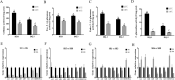
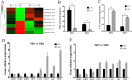
Low temperature is an important environmental factor that restricts the growth of Stropharia rugosoannulata; however, the molecular mechanisms underlying S. rugosoannulata responses to low-temperature stress are largely unknown. In this study, we performed a transcriptome analysis of a high-sensitivity strain (DQ-1) and low-sensitivity strain (DQ-3) under low-temperature stress. The liquid hyphae of S. rugosoannulata treated at 25 °C and 10 °C were analyzed by RNA-Seq, and a total of 9499 differentially expressed genes (DEGs) were identified. GO and KEGG enrichment analyses showed that these genes were enriched in "xenobiotic biodegradation and metabolism", "carbohydrate metabolism", "lipid metabolism" and "oxidoreductase activity". Further research found that carbohydrate enzyme (AA, GH, CE, and GT) genes were downregulated more significantly in DQ-1 than DQ-3 and several cellulase activities were also reduced to a greater extent. Moreover, the CAT1, CAT2, GR, and POD genes and more heat shock protein genes (HSP20, HSP78 and sHSP) were upregulated in the two strains after low-temperature stress, and the GPX gene and more heat shock protein genes were upregulated in DQ-3. In addition, the enzyme activity and qRT-PCR results showed trends similar to those of the RNA-Seq results. This result indicates that low-temperature stress reduces the expression of different AA, GH, CE, and GT enzyme genes and reduces the secretion of cellulase, thereby reducing the carbohydrate metabolism process and mycelial growth of S. rugosoannulata. Moreover, the expression levels of different types of antioxidant enzymes and heat shock proteins are also crucial for S. rugosoannulata to resist low-temperature stress. In short, this study will provide a basis for further research on important signaling pathways, gene functions and variety breeding of S. rugosoannulata related to low-temperature stress.
Keywords: Antioxidant enzyme; Carbohydrate enzymes; Heat shock protein; Low-temperature stress; Stropharia rugosoannulata; Transcriptomic.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Transcriptome and Differentially Expressed Gene Profiles in Mycelium, Primordium and Fruiting Body Development in Stropharia rugosoannulata.Genes (Basel). 2022 Jun 17;13(6):1080. doi: 10.3390/genes13061080. Genes (Basel). 2022. PMID: 35741841 Free PMC article.
-
Effects of Different Nitrogen Levels on Lignocellulolytic Enzyme Production and Gene Expression under Straw-State Cultivation in Stropharia rugosoannulata.Int J Mol Sci. 2023 Jun 13;24(12):10089. doi: 10.3390/ijms241210089. Int J Mol Sci. 2023. PMID: 37373235 Free PMC article.
-
Study of thermotolerant mechanism of Stropharia rugosoannulata under high temperature stress based on the transcriptome sequencing.Mycoscience. 2021 Mar 20;62(2):95-105. doi: 10.47371/mycosci.2020.11.006. eCollection 2021. Mycoscience. 2021. PMID: 37089254 Free PMC article.
-
Transcriptome Analysis Explored the Differential Genes' Expression During the Development of the Stropharia rugosoannulata Fruiting Body.Front Genet. 2022 Jun 29;13:924050. doi: 10.3389/fgene.2022.924050. eCollection 2022. Front Genet. 2022. PMID: 35903349 Free PMC article.
-
Nutritional and Therapeutic Potential of Stropharia rugosoannulata and Macrolepiota procera: From Composition to Health-Promoting Effect.J Fungi (Basel). 2025 Mar 27;11(4):259. doi: 10.3390/jof11040259. J Fungi (Basel). 2025. PMID: 40278080 Free PMC article. Review.
Cited by
-
Integrated analysis of transcriptomics and metabolomics of peach under cold stress.Front Plant Sci. 2023 Mar 27;14:1153902. doi: 10.3389/fpls.2023.1153902. eCollection 2023. Front Plant Sci. 2023. PMID: 37051086 Free PMC article.
-
Correction: Transcriptomic analysis of Stropharia rugosoannulata reveals carbohydrate metabolism and cold resistance mechanisms under low-temperature stress.AMB Express. 2022 Jun 13;12(1):72. doi: 10.1186/s13568-022-01412-y. AMB Express. 2022. PMID: 35697966 Free PMC article. No abstract available.
-
Genomic Analysis of Stropharia rugosoannulata Reveals Its Nutritional Strategy and Application Potential in Bioremediation.J Fungi (Basel). 2022 Feb 6;8(2):162. doi: 10.3390/jof8020162. J Fungi (Basel). 2022. PMID: 35205916 Free PMC article.
-
Transcriptomic, and metabolic profiling reveals adaptive mechanisms of Auricularia heimuer to temperature stress.PeerJ. 2025 Jul 21;13:e19713. doi: 10.7717/peerj.19713. eCollection 2025. PeerJ. 2025. PMID: 40708823 Free PMC article.
-
Comparative Transcriptome Profiles of the Response of Mycelia of the Genus Morchella to Temperature Stress: An Examination of Potential Resistance Mechanisms.J Fungi (Basel). 2024 Feb 27;10(3):178. doi: 10.3390/jof10030178. J Fungi (Basel). 2024. PMID: 38535187 Free PMC article.
References
-
- Collins GG, Xun LN, Saltveit ME. Heat shock proteins and chilling sensitivity of mung bean hypocotyls. J Exp Bot. 1995;7:795–802. doi: 10.1093/jxb/46.7.795. - DOI
-
- Dai YC, Yang ZL, Cui BK, Yu CJ, Zhou LW. Species diversity and utilization of medicinal mushrooms and fungi in China (Review) Int J Med Mushrooms. 2009;11:287–302. doi: 10.1615/IntJMedMushr.v11.i3.80. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

