Evaluation of commercial Anti-SARS-CoV-2 neutralizing antibody assays in seropositive subjects
- PMID: 35568003
- PMCID: PMC9044730
- DOI: 10.1016/j.jcv.2022.105169
Evaluation of commercial Anti-SARS-CoV-2 neutralizing antibody assays in seropositive subjects
Abstract
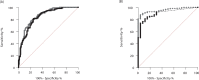
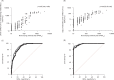
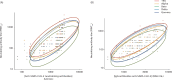
The virus neutralization test (VNT) is the reference for the assessment of the functional ability of neutralizing antibodies (NAb) to block SARS-CoV-2 entry into cells. New competitive immunoassays measuring antibodies preventing interaction between the spike protein and its cellular receptor are proposed as surrogate VNT (sVNT). We tested three commercial sVNT (a qualitative immunochromatographic test and two quantitative immunoassays named YHLO and TECO) together with a conventional anti-spike IgG assay (bioMérieux) in comparison with an in-house plaque reduction neutralization test (PRNT50) using the original 19A strain and different variants of concern (VOC), on a panel of 306 sera from naturally-infected or vaccinated patients. The qualitative test was rapidly discarded because of poor sensitivity and specificity. Areas under the curve of YHLO and TECO assays were, respectively, 85.83 and 84.07 (p-value >0.05) using a positivity threshold of 20 for PRNT50, and 95.63 and 90.35 (p-value =0.02) using a threshold of 80. However, the performances of YHLO and bioMérieux were very close for both thresholds, demonstrating the absence of added value of sVNT compared to a conventional assay for the evaluation of the presence of NAb in seropositive subjects. In addition, the PRNT50 assay showed a reduction of NAb titers towards different VOC in comparison to the 19A strain that could not be appreciated by the commercial tests. Despite the good correlation between the anti-spike antibody titer and the titer of NAb by PRNT50, our results highlight the difficulty to distinguish true NAb among the anti-RBD antibodies with commercial user-friendly immunoassays.
Keywords: Commercial tests; Competitive anti-RBD immunoassays; Neutralizing antibodies; SARS-CoV-2; Surrogate markers of protection.
Copyright © 2022. Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Bal A., Pozzetto B., Trabaud M.-.A., Escuret V., Rabilloud M., Langlois-Jacques C., et al. Evaluation of high-throughput SARS-CoV-2 serological assays in a longitudinal cohort of patients with mild COVID-19: clinical sensitivity, specificity, and association with virus neutralization test. Clin. Chem. 2021 Apr 29;67(5):742–752. - PMC - PubMed
-
- Legros V., Denolly S., Vogrig M., Boson B., Siret E., Rigaill J., et al. A longitudinal study of SARS-CoV-2-infected patients reveals a high correlation between neutralizing antibodies and COVID-19 severity. Cellular & Mol. Immunol. [Internet] 2021 Jan 6 [cited 2021 Jan 11]; Available from: http://www.nature.com/articles/s41423-020-00588-2. - PMC - PubMed
-
- Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020 May;581(7807):215–220. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

