Virological characteristics of the SARS-CoV-2 Omicron BA.2 spike
- PMID: 35568035
- PMCID: PMC9057982
- DOI: 10.1016/j.cell.2022.04.035
Virological characteristics of the SARS-CoV-2 Omicron BA.2 spike
Abstract
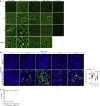
Soon after the emergence and global spread of the SARS-CoV-2 Omicron lineage BA.1, another Omicron lineage, BA.2, began outcompeting BA.1. The results of statistical analysis showed that the effective reproduction number of BA.2 is 1.4-fold higher than that of BA.1. Neutralization experiments revealed that immunity induced by COVID vaccines widely administered to human populations is not effective against BA.2, similar to BA.1, and that the antigenicity of BA.2 is notably different from that of BA.1. Cell culture experiments showed that the BA.2 spike confers higher replication efficacy in human nasal epithelial cells and is more efficient in mediating syncytia formation than the BA.1 spike. Furthermore, infection experiments using hamsters indicated that the BA.2 spike-bearing virus is more pathogenic than the BA.1 spike-bearing virus. Altogether, the results of our multiscale investigations suggest that the risk of BA.2 to global health is potentially higher than that of BA.1.
Keywords: B.1.1.529; BA.1; BA.2; COVID-19; Omicron; SARS-CoV-2; fusogenicity; pathogenicity; transmissibility.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

