Molecular basis for coordinating secondary metabolite production by bacterial and plant signaling molecules
- PMID: 35568198
- PMCID: PMC9163588
- DOI: 10.1016/j.jbc.2022.102027
Molecular basis for coordinating secondary metabolite production by bacterial and plant signaling molecules
Abstract
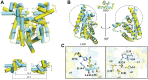
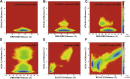
The production of secondary metabolites is a major mechanism used by beneficial rhizobacteria to antagonize plant pathogens. These bacteria have evolved to coordinate the production of different secondary metabolites due to the heavy metabolic burden imposed by secondary metabolism. However, for most secondary metabolites produced by bacteria, it is not known how their biosynthesis is coordinated. Here, we showed that PhlH from the rhizobacterium Pseudomonas fluorescens is a TetR-family regulator coordinating the expression of enzymes related to the biosynthesis of several secondary metabolites, including 2,4-diacetylphloroglucinol (2,4-DAPG), mupirocin, and pyoverdine. We present structures of PhlH in both its apo form and 2,4-DAPG-bound form and elucidate its ligand-recognizing and allosteric switching mechanisms. Moreover, we found that dissociation of 2,4-DAPG from the ligand-binding domain of PhlH was sufficient to allosterically trigger a pendulum-like movement of the DNA-binding domains within the PhlH dimer, leading to a closed-to-open conformational transition. Finally, molecular dynamics simulations confirmed that two distinct conformational states were stabilized by specific hydrogen bonding interactions and that disruption of these hydrogen bonds had profound effects on the conformational transition. Our findings not only reveal a well-conserved route of allosteric signal transduction in TetR-family regulators but also provide novel mechanistic insights into bacterial metabolic coregulation.
Keywords: 2,4-diacetylphloroglucinol; TetR-family transcriptional regulator; allosteric switching mechanism; bacterial metabolic coregulation; secondary metabolite.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures






Similar articles
-
Interspecies signaling modulates the biosynthesis of antimicrobial secondary metabolites related to biological control activities of Pseudomonas fluorescens 2P24.Microbiol Spectr. 2025 Mar 4;13(3):e0188624. doi: 10.1128/spectrum.01886-24. Epub 2025 Feb 3. Microbiol Spectr. 2025. PMID: 39898669 Free PMC article.
-
Pyoluteorin regulates the biosynthesis of 2,4-DAPG through the TetR family transcription factor PhlH in Pseudomonas protegens Pf-5.Appl Environ Microbiol. 2024 Apr 17;90(4):e0174323. doi: 10.1128/aem.01743-23. Epub 2024 Mar 12. Appl Environ Microbiol. 2024. PMID: 38470180 Free PMC article.
-
Transcriptional Regulator PhlH Modulates 2,4-Diacetylphloroglucinol Biosynthesis in Response to the Biosynthetic Intermediate and End Product.Appl Environ Microbiol. 2017 Oct 17;83(21):e01419-17. doi: 10.1128/AEM.01419-17. Print 2017 Nov 1. Appl Environ Microbiol. 2017. PMID: 28821548 Free PMC article.
-
Biosynthesis of phloroglucinol compounds in microorganisms--review.Appl Microbiol Biotechnol. 2012 Jan;93(2):487-95. doi: 10.1007/s00253-011-3712-6. Epub 2011 Nov 19. Appl Microbiol Biotechnol. 2012. PMID: 22101786 Review.
-
Studying allosteric regulation in metal sensor proteins using computational methods.Adv Protein Chem Struct Biol. 2014;96:181-218. doi: 10.1016/bs.apcsb.2014.06.009. Epub 2014 Sep 6. Adv Protein Chem Struct Biol. 2014. PMID: 25443958 Review.
Cited by
-
Interspecies signaling modulates the biosynthesis of antimicrobial secondary metabolites related to biological control activities of Pseudomonas fluorescens 2P24.Microbiol Spectr. 2025 Mar 4;13(3):e0188624. doi: 10.1128/spectrum.01886-24. Epub 2025 Feb 3. Microbiol Spectr. 2025. PMID: 39898669 Free PMC article.
-
Identification, Detection, and Management of Soft Rot Disease of Ginger in the Eastern Himalayan Region of India.Pathogens. 2025 May 29;14(6):544. doi: 10.3390/pathogens14060544. Pathogens. 2025. PMID: 40559552 Free PMC article.
-
Structural basis for spermidine recognition and modulation of Acinetobacter baumannii multidrug efflux regulator AmvR.mBio. 2025 May 14;16(5):e0008125. doi: 10.1128/mbio.00081-25. Epub 2025 Mar 31. mBio. 2025. PMID: 40162807 Free PMC article.
-
Pyoluteorin regulates the biosynthesis of 2,4-DAPG through the TetR family transcription factor PhlH in Pseudomonas protegens Pf-5.Appl Environ Microbiol. 2024 Apr 17;90(4):e0174323. doi: 10.1128/aem.01743-23. Epub 2024 Mar 12. Appl Environ Microbiol. 2024. PMID: 38470180 Free PMC article.
-
Production of the siderophore lysochelin in rich media through maltose-promoted high-density growth of Lysobacter sp. 3655.Front Microbiol. 2024 Jun 26;15:1433983. doi: 10.3389/fmicb.2024.1433983. eCollection 2024. Front Microbiol. 2024. PMID: 38989020 Free PMC article.
References
-
- Tengerdy R.P., Szakács G. Perspectives in agrobiotechnology. J. Biotechnol. 1998;66:91–99. - PubMed
-
- Haas D., Défago G. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat. Rev. Microbiol. 2005;3:307–319. - PubMed
-
- Siddiqui Z.A. Springer; Dordrecht: 2006. PGPR: Biocontrol and Biofertilization.
-
- Haas D., Keel C. Regulation of antibiotic production in root-colonizing Peudomonas spp. And relevance for biological control of plant disease. Annu. Rev. Phytopathol. 2003;41:117–153. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

