Evaluation of long-term antibody kinetics in healthcare workers vaccinated with inactivated COVID-19 Vero cell vaccine (CoronaVac), a propensity score-matched observational study
- PMID: 35568368
- PMCID: PMC9093161
- DOI: 10.1016/j.ijid.2022.05.007
Evaluation of long-term antibody kinetics in healthcare workers vaccinated with inactivated COVID-19 Vero cell vaccine (CoronaVac), a propensity score-matched observational study
Abstract
Objectives: This study aimed to evaluate the long-term antibody kinetics after vaccinating with an inactivated COVID-19 Vero cell vaccine (CoronaVac) in healthcare workers (HCWs) at a single center in Turkey.
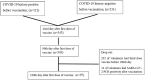
Methods: For this prospective observational study, Chemiluminescence immunoassay (CLIA) and enzyme-linked immunosorbent assay (ELISA) were used for the determination of binding antibodies (bAb) and neutralizing antibodies (nAb), respectively. Antibody kinetics were compared for the potential influencing factors, and propensity score analysis was performed to match the subcohort for age.
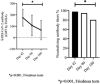
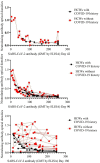
Results: Early bAb and nAb response was achieved in all 343 participants. Titers of bAbs against SARS-CoV-2 on 42 days post-vaccination (dpv) were higher in HCWs who were aged <40 years and who had a history of COVID-19. SARS-CoV-2 bAb levels in HCWs on days 42 (n = 97), 90 (n = 97), and 180 (n = 97) were 175 IU/ml (3.9-250), 107 IU/ml (2.4-250), and 66.1 IU/ml (2.57-250), respectively (p<0.001). SARS-CoV-2 bAb (p<0.001) and nAb (p<0.001) titers decreased significantly over time. There was a high negative correlation between SARS-CoV-2 antibody titers and inverse optic density of nAb responses (Pearson correlation coefficient: -0.738, p<0.001).
Conclusions: When the antibody responses were compared, it was seen that the vaccine immunogenicity was better in those who had prior COVID-19 history and were aged <40 years. In the course of time, it was determined that there was a significant decrease in bAb and nAb responses after the 90th day. These results may guide approval decisions for booster COVID-19 vaccines.
Copyright © 2022. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of competing interest The authors have no competing interests to declare.
Figures


Similar articles
-
Factors associated with neutralizing antibody levels induced by two inactivated COVID-19 vaccines for 12 months after primary series vaccination.Front Immunol. 2022 Sep 9;13:967051. doi: 10.3389/fimmu.2022.967051. eCollection 2022. Front Immunol. 2022. PMID: 36159863 Free PMC article.
-
A longitudinal study of humoral immune responses induced by a 3-dose inactivated COVID-19 vaccine in an observational, prospective cohort.BMC Immunol. 2022 Nov 16;23(1):57. doi: 10.1186/s12865-022-00532-1. BMC Immunol. 2022. PMID: 36384440 Free PMC article.
-
SARS-CoV-2 specific antibody responses in healthcare workers after a third booster dose of CoronaVac or BNT162b2 vaccine.J Med Virol. 2022 Aug;94(8):3768-3775. doi: 10.1002/jmv.27794. Epub 2022 Apr 23. J Med Virol. 2022. PMID: 35434796 Free PMC article.
-
Post COVID-19 vaccination binding and neutralizing antibody with or without previous infection: An 18-month longitudinal study in Indonesia.Narra J. 2024 Aug;4(2):e1071. doi: 10.52225/narra.v4i2.1071. Epub 2024 Aug 31. Narra J. 2024. PMID: 39280276 Free PMC article.
-
Immunogenicity after two doses of inactivated virus vaccine in healthcare workers with and without previous COVID-19 infection: Prospective observational study.J Med Virol. 2022 Jan;94(1):279-286. doi: 10.1002/jmv.27316. Epub 2021 Sep 10. J Med Virol. 2022. PMID: 34468990 Free PMC article.
Cited by
-
Safety and immunogenicity of CoronaVac in healthy adults: A prospective observational multicenter real-world study in Henan Province, China.Virulence. 2024 Dec;15(1):2310450. doi: 10.1080/21505594.2024.2310450. Epub 2024 Feb 7. Virulence. 2024. PMID: 38326274 Free PMC article.
References
-
- Centers for Disease Control and Prevention . 2022. COVID-19 (SARS-CoV-2 Infection) Laboratory Biosafety Guide.https://www.cdc.gov/coronavirus/2019-ncov/lab/lab-biosafety-guidelines.html (Accessed 24 Jan 2022)
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

