Covid-19 vaccine immunogenicity in people living with HIV-1
- PMID: 35568588
- PMCID: PMC9069249
- DOI: 10.1016/j.vaccine.2022.04.090
Covid-19 vaccine immunogenicity in people living with HIV-1
Abstract
Introduction: COVID-19 vaccine efficacy has been evaluated in large clinical trials and in real-world situation. Although they have proven to be very effective in the general population, little is known about their efficacy in immunocompromised patients. HIV-infected individuals' response to vaccine may vary according to the type of vaccine and their level of immunosuppression. We evaluated immunogenicity of an mRNA anti-SARS CoV-2 vaccine in HIV-positive individuals.
Methods: HIV-positive individuals (n = 121) were recruited from HIV clinics in Montreal and stratified according to their CD4 counts. A control group of 20 health care workers naïve to SARS CoV-2 was used. The participants' Anti-RBD IgG responses were measured by ELISA at baseline and 3-4 weeks after receiving the first dose of an mRNA vaccine).
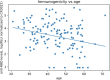
Results: Eleven of 121 participants had anti-COVID-19 antibodies at baseline, and a further 4 had incomplete data for the analysis. Mean anti-RBD IgG responses were similar between the HIV negative control group (n = 20) and the combined HIV+ group (n = 106) (p = 0.72). However, these responses were significantly lower in the group with <250 CD4 cells/mm3. (p < 0.0001). Increasing age was independently associated with decreased immunogenicity.
Conclusion: HIV-positive individuals with CD4 counts over 250 cells/mm3 have an anti-RBD IgG response similar to the general population. However, HIV-positive individuals with the lowest CD4 counts (<250 cells/mm3) have a weaker response. These data would support the hypothesis that a booster dose might be needed in this subgroup of HIV-positive individuals, depending on their response to the second dose.
Keywords: Covid-19 Vaccine immunogenicity; Covid-19 Vaccines in Immunocompromised Patients; Covid-19 Vaccines in people living with HIV; Vaccine immunogenicity HIV; mRNA Vaccines in people living with HIV; mRNA vaccine immunogenicity in people living with HIV.
Copyright © 2022 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. - PMC - PubMed
-
- Paris C., Perrin S., Hamonic S., Bourget B., Roué C., Brassard O., et al. Effectiveness of mRNA-BNT162b2, mRNA-1273, and ChAdOx1 nCoV-19 vaccines against COVID-19 in health care workers: an observational study using surveillance data. Clin Microbiol Infect. 2021;27(11):1699.e5–1699.e8. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

