A first-in-human Phase I dose-escalation trial of the novel therapeutic peptide, ALM201, demonstrates a favourable safety profile in unselected patients with ovarian cancer and other advanced solid tumours
- PMID: 35568736
- PMCID: PMC9276671
- DOI: 10.1038/s41416-022-01780-z
A first-in-human Phase I dose-escalation trial of the novel therapeutic peptide, ALM201, demonstrates a favourable safety profile in unselected patients with ovarian cancer and other advanced solid tumours
Abstract
Background: We aimed to assess the safety, tolerability and pharmacokinetics of a novel anti-angiogenic peptide.
Methods: We used an open-label, multicentre, dose-escalation Phase I trial design in patients with solid tumours. ALM201 was administered subcutaneously once daily for 5 days every week in unselected patients with solid tumours.
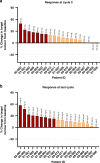
Results: Twenty (8 male, 12 female) patients with various solid tumours were treated (18 evaluable for toxicity) over eight planned dose levels (10-300 mg). ALM201 was well-tolerated at all dose levels without CTCAE grade 4 toxicities. Adverse events were predominantly grades 1-2, most commonly, localised injection-site reactions (44.4%), vomiting (11%), fatigue (16.7%), arthralgia (5.6%) and headache (11%). Thrombosis occurred in two patients at the 100 mg and 10 mg dose levels. The MTD was not reached, and a recommended Phase II dose (RP2D) based on feasibility was declared. Plasma exposure increased with dose (less than dose-proportional at the two highest dose levels). No peptide accumulation was evident. The median treatment duration was 11.1 (range 3-18) weeks. Four of 18 evaluable patients (22%) had stable disease.
Conclusions: Doses up to 300 mg of ALM201 subcutaneously are feasible and well-tolerated. Further investigation of this agent in selected tumour types/settings would benefit from patient-selection biomarkers.
© 2022. The Author(s).
Conflict of interest statement
Dr. AEH was awarded a travel bursary from Almac Discovery to present at ESMO 2017. Professor Richard Wilson received funding from Almac Discovery in 2014–2017 to support a clinical research fellowship. Professor RK is employed by Almac as the Medical Director. Dr. ANC is an employee of Almac Discovery. Dr. HS was paid a consultancy fee for advice and help with data analysis. The remaining authors declare no competing interests.
Figures


Similar articles
-
Evorpacept alone and in combination with pembrolizumab or trastuzumab in patients with advanced solid tumours (ASPEN-01): a first-in-human, open-label, multicentre, phase 1 dose-escalation and dose-expansion study.Lancet Oncol. 2021 Dec;22(12):1740-1751. doi: 10.1016/S1470-2045(21)00584-2. Epub 2021 Nov 15. Lancet Oncol. 2021. PMID: 34793719 Clinical Trial.
-
Rogaratinib in patients with advanced cancers selected by FGFR mRNA expression: a phase 1 dose-escalation and dose-expansion study.Lancet Oncol. 2019 Oct;20(10):1454-1466. doi: 10.1016/S1470-2045(19)30412-7. Epub 2019 Aug 9. Lancet Oncol. 2019. PMID: 31405822 Clinical Trial.
-
Pamiparib in combination with tislelizumab in patients with advanced solid tumours: results from the dose-escalation stage of a multicentre, open-label, phase 1a/b trial.Lancet Oncol. 2019 Sep;20(9):1306-1315. doi: 10.1016/S1470-2045(19)30396-1. Epub 2019 Aug 1. Lancet Oncol. 2019. PMID: 31378459 Clinical Trial.
-
Phase I dose-escalation studies of roniciclib, a pan-cyclin-dependent kinase inhibitor, in advanced malignancies.Br J Cancer. 2017 Jun 6;116(12):1505-1512. doi: 10.1038/bjc.2017.92. Epub 2017 May 2. Br J Cancer. 2017. PMID: 28463960 Free PMC article. Clinical Trial.
-
Phase 1 dose-escalation study of mirvetuximab soravtansine (IMGN853), a folate receptor α-targeting antibody-drug conjugate, in patients with solid tumors.Cancer. 2017 Aug 15;123(16):3080-3087. doi: 10.1002/cncr.30736. Epub 2017 Apr 25. Cancer. 2017. PMID: 28440955 Free PMC article. Clinical Trial.
Cited by
-
The FKBPL-based therapeutic peptide, AD-01, protects the endothelium from hypoxia-induced damage by stabilising hypoxia inducible factor-α and inflammation.J Transl Med. 2025 Mar 11;23(1):309. doi: 10.1186/s12967-025-06118-w. J Transl Med. 2025. PMID: 40069829 Free PMC article.
-
Successful Proof-of-Concept for Topical Delivery of Novel Peptide ALM201 with Potential Usefulness for Treating Neovascular Eye Disorders.Ophthalmol Sci. 2022 Apr 4;2(2):100150. doi: 10.1016/j.xops.2022.100150. eCollection 2022 Jun. Ophthalmol Sci. 2022. PMID: 36249680 Free PMC article.
-
Chitosan alchemy: transforming tissue engineering and wound healing.RSC Adv. 2024 Jun 17;14(27):19219-19256. doi: 10.1039/d4ra01594k. eCollection 2024 Jun 12. RSC Adv. 2024. PMID: 38887635 Free PMC article. Review.
-
Loss of Expression of Antiangiogenic Protein FKBPL in Endometrioid Endometrial Carcinoma: Implications for Clinical Practice.Medicina (Kaunas). 2022 Sep 22;58(10):1330. doi: 10.3390/medicina58101330. Medicina (Kaunas). 2022. PMID: 36295491 Free PMC article.
-
Evaluating oxidative stress targeting treatments in in vitro models of placental stress relevant to preeclampsia.Front Cell Dev Biol. 2025 Feb 28;13:1539496. doi: 10.3389/fcell.2025.1539496. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40109359 Free PMC article.

