The sham effect of invasive interventions in chronic coronary syndromes: a systematic review and meta-analysis
- PMID: 35568808
- PMCID: PMC9107755
- DOI: 10.1186/s12872-022-02658-x
The sham effect of invasive interventions in chronic coronary syndromes: a systematic review and meta-analysis
Abstract
Background: Some patients with chronic coronary syndromes undergo invasive procedures but the efficacy of such interventions remains to be robustly established by randomised sham-controlled trials (RCTs).
Purpose: To determine the sham effect in patients with chronic coronary syndromes enrolled in RCTs by performing a systematic review and meta-analysis.
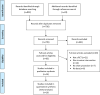
Methods: In April 2022, we performed a literature search for published patient-blind RCTs (CENTRAL, MEDLINE®, PsycINFO, and reference lists) with sham procedures, reporting the pre-post effects in the invasive sham arm among patients with Canadian cardiovascular society (CCS) angina or angina equivalents.
Results: 16 RCTs were included with 546 patients in the sham arm. Pooled results showed that sham interventions were associated with: improvement of 7% (95% CI 2-11%; I2 = 0%) in exercise time; decrease of 0.78 (95% CI - 1.10 to - 0.47; I2 = 75%) in CCS angina class; decrease of 53% (95% CI 24-71%; I2 = 96%) and 25% (95% CI 20-29%; I2 = 0%) in anginal episodes and nitroglycerine (NTG) use, respectively. Pooled results also showed an improvement in the physical functioning, angina frequency, treatment satisfaction, and disease perception domains of the Seattle Angina Questionnaire (SAQ).
Conclusion: Sham interventions in patients with chronic coronary syndromes were associated with a significant decrease in anginal episodes, NTG use, and CCS angina class and increased SAQ quality of life and exercise time. These results highlight the need for previous non sham-controlled trials to be interpreted with caution, and the importance of new invasive interventions to be evaluated versus a sham procedure.
Keywords: Chronic coronary syndromes; Invasive treatment; Sham effect; Sham procedure.
© 2022. The Author(s).
Conflict of interest statement
DC has participated in educational meetings and/or attended conferences or symposia (including travel, accommodation, and/or hospitality) with Bristol-Myers Squibb, Bayer, Boehringer Ingelheim, Daiichi Sankyo, Merck Serono, Ferrer, Pfizer, Novartis, and Roche > 3 years; no conflicts of interest < 3 years. JJF had speaker and consultant fees with Grünenthal, Fundação MSD (Portugal), TEVA, MSD, Allergan, Medtronic, GlaxoSmithKline, Novartis, Lundbeck, Solvay, BIAL, Merck Serono, Merz, Ipsen, Biogen, Acadia, Allergan, Abbvie, Sunovion-Pharmaceuticals. FJP had consultant and speaker fees with Astra Zeneca, Bayer, BMS, Boehringer Ingelheim and Daiichi Sankyo. There is no any other conflict of interest by the rest of the authors.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

