Anxiety-like behavior and anxiolytic treatment in the Rett syndrome natural history study
- PMID: 35568815
- PMCID: PMC9107202
- DOI: 10.1186/s11689-022-09432-2
Anxiety-like behavior and anxiolytic treatment in the Rett syndrome natural history study
Abstract
Background: Rett syndrome (RTT) is a neurodevelopmental disorder most often related to a pathogenic variant in the X-linked MECP2 gene. Internalizing behaviors appear to be common, but standard methods of diagnosing anxiety are not readily applied in this population which typically has cognitive impairment and limited expressive language. This study aims to describe the frequency of anxiety-like behavior and anxiolytic treatments along with associated clinical features in individuals with RTT.
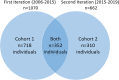
Methods: Parental reports and medication logs provided data from 1380 females with RTT participating in two iterations of the multicenter U.S. RTT Natural History Study (RNHS) from 2006 to 2019.
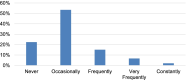
Results: Most participants with RTT (77.5%) had at least occasional anxious or nervous behavior. Anxiety was reported to be the most troublesome concern for 2.6%, and within the top 3 concerns for 10.0%, of participants in the second iteration. Parents directly reported treatment for anxious or nervous behavior in 16.6% of participants in the second iteration with most reporting good control of the behavior (71.6%). In the medication logs of both RNHS iterations, the indication of anxiety was listed for a similar number of participants (15% and 14.5%, respectively). Increased use of anxiolytics and selective serotonin reuptake inhibitors (SSRIs) was related to more frequent anxiety-like behaviors (P < 0.001), older age (P < 0.001), and mild MECP2 variants (P = 0.002).
Conclusion: Anxiety-like behavior is frequent at all ages and is a significant parental concern in RTT. Older individuals and those with mild MECP2 variants are more likely to be treated with medications. Better diagnosis and treatment of anxiety in RTT should be a goal of both future studies and clinical care.
Trial registration: NCT00299312 and NCT02738281.
Keywords: Anti-anxiety agents; Anxiety; Methyl-CpG-binding protein 2; Natural history studies; Rett syndrome.
© 2022. The Author(s).
Conflict of interest statement
Caroline B. Buchanan received funding from Rettsyndrome.org (Mentored Clinical Fellowship under U54 HD 061222 grant) and has done clinical trials with Acadia, Anavex, GW Pharmaceuticals, and Zynerba. Timothy A. Benke received funding from the NIH (U54 HD061222, R21 NS101288, R01 NS081248), International Foundation for CDKL5 Research, Loulou Foundation, and Children’s Hospital Colorado Foundation Ponzio Family Chair in Neurology Research. He is also a consultant for AveXis, Ovid, GW Pharmaceuticals, International Rett Syndrome Foundation, Takeda, and Marinus and has done clinical trials with Acadia, Ovid, GW Pharmaceuticals, Marinus, and Rett Syndrome Research Trust; all remuneration has been made to his department. Daniel G Glaze received funding from the NIH, Rettsyndrome.org, and the Blue Bird Circle. He has done clinical trials with Acadia, Neuren, and GW Pharmaceuticals. Richard H. Haas received funding from the NIH 5U54HD061222 grant and has done clinical trials sponsored by Acadia, GW Pharmaceuticals, and Newron Pharmaceuticals. Jane B. Lane received funding from the NIH and is a consultant for International Rett Syndrome Foundation and GW Pharmaceuticals. David N. Lieberman has done clinical trials with Anavex, Acadia, GW Pharmaceuticals, and Rett Syndrome Research Trust. Eric D. Marsh received funding from Rett Syndrome Research Trust, NIH, State of Pennsylvania, Penn Orphan Disease Center, International Rett Syndrome Foundation, LouLou Foundation, and Eagles Autism Foundation. He is a consultant for Cilpa Therapeutics and Stoke Therapeutics. He has done clinical trials with Zogenix, GW Pharmaceuticals, Acadia, Marinus, Stoke Therapeutics, and Rett Syndrome Research Trust. Jeffrey L. Neul received funding from the NIH and is a consultant for AveXis, Biohaven, Eloxx Pharmaceuticals, Ovid, Takeda, and Teva Pharmaceuticals. He has done clinical trials with Acadia and Newron Pharmaceuticals. Sarika U. Peters received funding from NIH, Rettsyndrome.org and is a consultant for Acadia Pharmaceuticals. Steve A. Skinner has done clinical trials with Acadia, Anavex, and GW Pharmaceuticals. Shannon M. Standridge is a speaker for Greenwich Biosciences and Scientific Advisory Board of Acadia. Walter E. Kaufmann received funding from the NIH and CDC, and is a consultant for Anavex, AveXis, Acadia, EryDel, Newron, GW Pharmaceuticals, Marinus, Biohaven, Zynerba, Ovid Therapeutics, and Stalicla. Currently, he is Chief Medical Officer of Anavex Life Sciences Corp. Alan K. Percy received funding from the NIH and is a consultant for Anavex, AveXis, Acadia, and GW Pharmaceuticals. He has done clinical trials with Anavex, Acadia, GW Pharmaceuticals, and Rett Syndrome Research Trust. The rest of the authors, Jennifer L. Stallworth, Aubin E. Joy, Rebekah E. Dixon, Alexandra E. Scott, Arthur A. Beisang, Peter T. Heydemann, Mary D. Jones, and Robin C. Ryther, declare that they have no competing interests.
Figures



References
-
- Bebbington A, Anderson A, Ravine D, Fyfe S, Pineda M, de Klerk N, Leonard H. Investigating genotype-phenotype relationships in Rett syndrome using an international data set. Neurology. 2008;70(11):868–875. doi: 10.1212/01.wnl.0000304752.50773.ec. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

