Incidence and immunomic features of apyretic COVID-19 in patients affected by solid tumors: a prospective cohort study
- PMID: 35568887
- PMCID: PMC9107211
- DOI: 10.1186/s12967-022-03429-0
Incidence and immunomic features of apyretic COVID-19 in patients affected by solid tumors: a prospective cohort study
Abstract
Background and rationale: Little is known about SARS-CoV-2 seroconversion in asymptomatic patients affected by solid cancer, and whether it is associated with specific transcriptomics changes in peripheral blood mononuclear cells (PBMC).
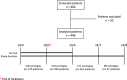
Methods: Patients affected by solid cancer treated in a top comprehensive cancer center in Italy during the first COVID-19 pandemic wave, and negative for COVID-19-symptoms since the first detection of COVID-19 in Italy, were prospectively evaluated by SARS-CoV-2 serology in the period between April 14th and June 23rd 2020. Follow-up serologies were performed, every 21-28 days, until August 23rd 2020. All SARS-CoV-2 IgM + patients underwent confirmatory nasopharyngeal swab (NPS). PBMCs from a subset of SARS-CoV-2 IgM + patients were collected at baseline, at 2 months, and at 7 months for transcriptome sequencing.
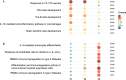
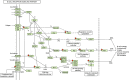
Results: SARS-CoV-2 serology was performed on 446 of the 466 recruited patients. A total of 14 patients (3.14%) tested positive for at least one SARS-CoV-2 immunoglobulin in the period between April 14th and August 23rd 2020. Incidence of SARS-CoV-2 IgM decreased from 1.48% in the first month of the accrual to 0% in the last month. Viral RNA could not be detected in any of the NPS. PBMC serial transcriptomic analysis showed progressive downregulation of interleukin 6 upregulated signatures, chemokine-mediated signaling and chemokine-chemokine receptor KEGG pathways. B- and T-cell receptor pathways (p-values = 0.0002 and 0.017 respectively) were progressively upregulated.
Conclusions: SARS-CoV-2 seroconversion rate in asymptomatic patients affected by solid cancer is consistent with that of asymptomatic COVID-19 assessed in the general population through NPS at the peak of the first wave. Transcriptomic features over time in IgM + asymptomatic cases are suggestive of previous viral exposure.
Keywords: Asymptomatic infection; COVID-19; Cancer; Serology; Transcriptome sequencing.
© 2022. The Author(s).
Conflict of interest statement
GZ received consulting fees from Novartis, travel grants from Novartis, Pfizer, and Roche, and research reagents from Cytiva and ThermoFisher Scientific. The other Authors report no conflict of interest.
Figures
Similar articles
-
Detection of SARS-CoV-2 infection prevalence in 860 cancer patients with a combined screening procedure including triage, molecular nasopharyngeal swabs and rapid serological test. A report from the first epidemic wave.PLoS One. 2022 Feb 2;17(2):e0262784. doi: 10.1371/journal.pone.0262784. eCollection 2022. PLoS One. 2022. PMID: 35108300 Free PMC article.
-
The SARS-CoV-2 Ivermectin Navarra-ISGlobal Trial (SAINT) to Evaluate the Potential of Ivermectin to Reduce COVID-19 Transmission in low risk, non-severe COVID-19 patients in the first 48 hours after symptoms onset: A structured summary of a study protocol for a randomized control pilot trial.Trials. 2020 Jun 8;21(1):498. doi: 10.1186/s13063-020-04421-z. Trials. 2020. PMID: 32513289 Free PMC article.
-
[Seroprevalence of anti-SARS-CoV-2 IgG/IgM antibodies in Borgosesia (Piedmont Region, Northern Italy) population: a surveillance strategy in post-lockdown period?].Epidemiol Prev. 2020 Sep-Dec;44(5-6 Suppl 2):200-206. doi: 10.19191/EP20.5-6.S2.119. Epidemiol Prev. 2020. PMID: 33412811 Italian.
-
Seroprevalence of SARS-CoV-2-specific antibodies in cancer outpatients in Madrid (Spain): A single center, prospective, cohort study and a review of available data.Cancer Treat Rev. 2020 Nov;90:102102. doi: 10.1016/j.ctrv.2020.102102. Epub 2020 Sep 1. Cancer Treat Rev. 2020. PMID: 32947121 Free PMC article. Review.
-
Seropositivity rate and diagnostic accuracy of serological tests in 2019-nCoV cases: a pooled analysis of individual studies.Eur Rev Med Pharmacol Sci. 2020 Oct;24(19):10208-10218. doi: 10.26355/eurrev_202010_23243. Eur Rev Med Pharmacol Sci. 2020. PMID: 33090430 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

