Metabolic engineering of Clostridium ljungdahlii for the production of hexanol and butanol from CO2 and H2
- PMID: 35568911
- PMCID: PMC9107641
- DOI: 10.1186/s12934-022-01802-8
Metabolic engineering of Clostridium ljungdahlii for the production of hexanol and butanol from CO2 and H2
Abstract
Background: The replacement of fossil fuels and petrochemicals with sustainable alternatives is necessary to mitigate the effects of climate change and also to counteract diminishing fossil resources. Acetogenic microorganisms such as Clostridium spp. are promising sources of fuels and basic chemical precursors because they efficiently utilize CO and CO2 as carbon source. However the conversion into high titers of butanol and hexanol is challenging.
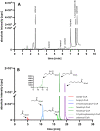
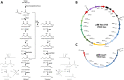
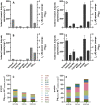
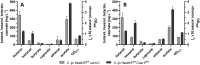
Results: Using a metabolic engineering approach we transferred a 17.9-kb gene cluster via conjugation, containing 13 genes from C. kluyveri and C. acetobutylicum for butanol and hexanol biosynthesis, into C. ljungdahlii. Plasmid-based expression resulted in 1075 mg L-1 butanol and 133 mg L-1 hexanol from fructose in complex medium, and 174 mg L-1 butanol and 15 mg L-1 hexanol from gaseous substrate (20% CO2 and 80% H2) in minimal medium. Product formation was increased by the genomic integration of the heterologous gene cluster. We confirmed the expression of all 13 enzymes by targeted proteomics and identified potential rate-limiting steps. Then, we removed the first-round selection marker using CRISPR/Cas9 and integrated an additional 7.8 kb gene cluster comprising 6 genes from C. carboxidivorans. This led to a significant increase in the hexanol titer (251 mg L-1) at the expense of butanol (158 mg L-1), when grown on CO2 and H2 in serum bottles. Fermentation of this strain at 2-L scale produced 109 mg L-1 butanol and 393 mg L-1 hexanol.
Conclusions: We thus confirmed the function of the butanol/hexanol biosynthesis genes and achieved hexanol biosynthesis in the syngas-fermenting species C. ljungdahlii for the first time, reaching the levels produced naturally by C. carboxidivorans. The genomic integration strain produced hexanol without selection and is therefore suitable for continuous fermentation processes.
Keywords: Acetogens; Biofuels; Butanol; Hexanol; Syngas fermentation; Wood-Ljungdahl pathway.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Vojtisek-Lom M, Beránek V, Mikuška P, Křůmal K, Coufalík P, Sikorová J, Topinka J. Blends of butanol and hydrotreated vegetable oils as drop-in replacement for diesel engines: effects on combustion and emissions. Fuel. 2017;197:407–421. doi: 10.1016/j.fuel.2017.02.039. - DOI
-
- De Poures MV, Sathiyagnanam AP, Rana D, Rajesh Kumar B, Saravanan S. 1-Hexanol as a sustainable biofuel in DI diesel engines and its effect on combustion and emissions under the influence of injection timing and exhaust gas recirculation (EGR) Appl Therm Eng. 2017;113:1505–1513. doi: 10.1016/j.applthermaleng.2016.11.164. - DOI
-
- Breitkreuz K, Menne A, Kraft A. New process for sustainable fuels and chemicals from bio-based alcohols and acetone. Biofuels Bioprod Biorefin. 2014;8:504–515. doi: 10.1002/bbb.1484. - DOI
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

