High-fat diet exacerbates cognitive decline in mouse models of Alzheimer's disease and mixed dementia in a sex-dependent manner
- PMID: 35568928
- PMCID: PMC9107741
- DOI: 10.1186/s12974-022-02466-2
High-fat diet exacerbates cognitive decline in mouse models of Alzheimer's disease and mixed dementia in a sex-dependent manner
Abstract
Background: Approximately 70% of Alzheimer's disease (AD) patients have co-morbid vascular contributions to cognitive impairment and dementia (VCID); this highly prevalent overlap of dementia subtypes is known as mixed dementia (MxD). AD is more prevalent in women, while VCID is slightly more prevalent in men. Sex differences in risk factors may contribute to sex differences in dementia subtypes. Unlike metabolically healthy women, diabetic women are more likely to develop VCID than diabetic men. Prediabetes is 3× more prevalent than diabetes and is linked to earlier onset of dementia in women, but not men. How prediabetes influences underlying pathology and cognitive outcomes across different dementia subtypes is unknown. To fill this gap in knowledge, we investigated the impact of diet-induced prediabetes and biological sex on cognitive function and neuropathology in mouse models of AD and MxD.
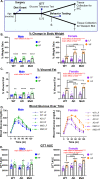
Methods: Male and female 3xTg-AD mice received a sham (AD model) or unilateral common carotid artery occlusion surgery to induce chronic cerebral hypoperfusion (MxD model). Mice were fed a control or high fat (HF; 60% fat) diet from 3 to 7 months of age. In both sexes, HF diet elicited a prediabetic phenotype (impaired glucose tolerance) and weight gain.
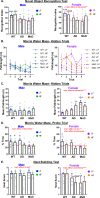
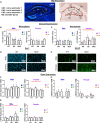
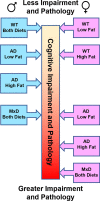
Results: In females, but not males, metabolic consequences of a HF diet were more severe in AD or MxD mice compared to WT. In both sexes, HF-fed AD or MxD mice displayed deficits in spatial memory in the Morris water maze (MWM). In females, but not males, HF-fed AD and MxD mice also displayed impaired spatial learning in the MWM. In females, but not males, AD or MxD caused deficits in activities of daily living, regardless of diet. Astrogliosis was more severe in AD and MxD females compared to males. Further, AD/MxD females had more amyloid beta plaques and hippocampal levels of insoluble amyloid beta 40 and 42 than AD/MxD males. In females, but not males, more severe glucose intolerance (prediabetes) was correlated with increased hippocampal microgliosis.
Conclusions: High-fat diet had a wider array of metabolic, cognitive, and neuropathological consequences in AD and MxD females compared to males. These findings shed light on potential underlying mechanisms by which prediabetes may lead to earlier dementia onset in women.
Keywords: Alzheimer’s disease; Cerebral hypoperfusion; Dementia; Diabetes; Glucose intolerance; High-fat diet; Inflammation; Metabolic; Obesity; Sex; Vascular.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


References
-
- Chatterjee S, Peters SA, Woodward M, MejiaArango S, Batty GD, Beckett N, et al. Type 2 diabetes as a risk factor for dementia in women compared with men: a pooled analysis of 23 million people comprising more than 100,000 cases of dementia. Diabetes Care. 2016;39(2):300–307. doi: 10.2337/dc15-1588. - DOI - PMC - PubMed
-
- Gottesman RF, Albert MS, Alonso A, Coker LH, Coresh J, Davis SM, et al. Associations between midlife vascular risk factors and 25-year incident dementia in the atherosclerosis risk in communities (ARIC) Cohort. JAMA Neurol. 2017;74(10):1246–1254. doi: 10.1001/jamaneurol.2017.1658. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

