Sleep Fragmentation and Estradiol Suppression Decrease Fat Oxidation in Premenopausal Women
- PMID: 35569055
- PMCID: PMC9282266
- DOI: 10.1210/clinem/dgac313
Sleep Fragmentation and Estradiol Suppression Decrease Fat Oxidation in Premenopausal Women
Abstract
Context: Body fat gain associated with menopause has been attributed to estradiol (E2) withdrawal. Hypoestrogenism is unlikely to be the only contributing factor, however.
Objective: Given the links between sleep and metabolic health, we examined the effects of an experimental menopausal model of sleep fragmentation on energy metabolism.
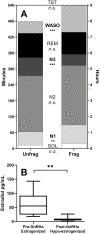
Methods: Twenty premenopausal women (age 21-45 years) underwent a 5-night inpatient study during the mid-to-late follicular phase (estrogenized; n = 20) and the same protocol was repeated in a subset of the participants (n = 9) following leuprolide-induced E2 suppression (hypo-estrogenized). During each 5-night study, there were 2 nights of unfragmented sleep followed by 3 nights of fragmented sleep. Indirect calorimetry was used to assess fasted resting energy expenditure (REE) and substrate oxidation.
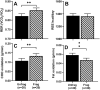
Results: Sleep fragmentation in the estrogenized state increased the respiratory exchange ratio (RER) and carbohydrate oxidation while decreasing fat oxidation (all P < 0.01). Similarly, in the hypo-estrogenized state without sleep fragmentation, RER and carbohydrate oxidation increased and fat oxidation decreased (all P < 0.01); addition of sleep fragmentation to the hypo-estrogenized state did not produce further effects beyond that observed for either intervention alone (P < 0.05). There were no effects of either sleep fragmentation or E2 state on REE.
Conclusion: Sleep fragmentation and hypoestrogenism each independently alter fasting substrate oxidation in a manner that may contribute to body fat gain. These findings are important for understanding mechanisms underlying propensity to body fat gain in women across the menopause transition.
Keywords: estradiol; indirect calorimetry; menopause; sleep fragmentation; substrate oxidation; women.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Endocrine Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures