Neoadjuvant Chemotherapy and Nodal Response Rates in Luminal Breast Cancer: Effects of Age and Tumor Ki67
- PMID: 35569077
- PMCID: PMC9466990
- DOI: 10.1245/s10434-022-11871-z
Neoadjuvant Chemotherapy and Nodal Response Rates in Luminal Breast Cancer: Effects of Age and Tumor Ki67
Abstract
Background: Neoadjuvant chemotherapy (NAC) is standard for most triple-negative and human epidermal growth factor receptor 2 (HER2)+ breast cancers, and frequently downstages node-positive (cN+) disease, permitting omission of axillary dissection. In estrogen receptor (ER)+/HER2- disease, response rates are lower. Whether Ki67 is associated with axillary downstaging in ER+/HER2- disease is unknown.
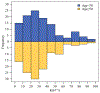
Methods: With institutional review board approval, we queried our institutional database to identify all patients with primary ER+/HER2- biopsy-proven cN+ breast cancer treated with NAC followed by surgery from January 2012 to December 2021. Nodal pathologic complete response (pCR) rates were evaluated by pretreatment Ki67 and patient age.
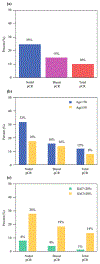
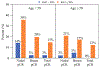
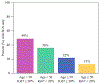
Results: 315 patients (median age 50 years) were included. Nodal pCR rate was 24.8% (78/315) and was higher in patients aged < 50 years than ≥ 50 years (31.8% versus 17.7%, p = 0.004). Ki67 was available on 236 patients (74.9%). Median Ki67 was 29.0% (range 1-98%) and did not differ by age category (p = 0.23). Patients with nodal pCR had higher Ki67 (median 40.3% versus 25.0%, p < 0.001). Nodal pCR rates were 28.4% (Ki67 ≥ 20%) versus 8.1% (Ki67 < 20%) (p < 0.001). On multivariable analysis, Ki67 and age category were predictive of nodal pCR. Combining these two parameters together, nodal pCR rates in age < 50 years were 35.8% when Ki67 ≥ 20% versus 14.3% with Ki67 < 20% (p = 0.02). In contrast, for age ≥ 50 years, nodal pCR was 21.0% for Ki67 ≥ 20% versus 2.6% with Ki67 < 20% (p = 0.008).
Conclusions: In ER+/HER2- breast cancer, nodal downstaging with NAC is associated with age (< 50 years) and Ki67 (≥ 20%). Age and Ki67 should be considered for NAC decision-making and can identify patients with high rates of nodal downstaging (36%) who would benefit from NAC to enable axillary preservation.
© 2022. Society of Surgical Oncology.
Figures
References
-
- Murphy BL, Day CN, Hoskin TL, Habermann EB, Boughey JC. Neoadjuvant chemotherapy use in breast cancer is greatest in excellent responders: triple-negative and HER2+ subtypes. Ann Surg Oncol. 2018;25(8):2241–8. - PubMed
-
- Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–72. - PubMed
-
- Boughey JC, McCall LM, Ballman KV, et al. Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (Alliance) prospective multicenter clinical trial. Ann Surg. 2014;260(4):608–14 (discussion 614-6). - PMC - PubMed
-
- Harbeck N, Rastogi P, Martin M, et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: updated efficacy and Ki-67 analysis from the MonarchE study. Ann Oncol. 2021;32(12):1571–81. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

