Effect of Robot-Assisted Radical Cystectomy With Intracorporeal Urinary Diversion vs Open Radical Cystectomy on 90-Day Morbidity and Mortality Among Patients With Bladder Cancer: A Randomized Clinical Trial
- PMID: 35569079
- PMCID: PMC9109000
- DOI: 10.1001/jama.2022.7393
Effect of Robot-Assisted Radical Cystectomy With Intracorporeal Urinary Diversion vs Open Radical Cystectomy on 90-Day Morbidity and Mortality Among Patients With Bladder Cancer: A Randomized Clinical Trial
Abstract
Importance: Robot-assisted radical cystectomy is being performed with increasing frequency, but it is unclear whether total intracorporeal surgery improves recovery compared with open radical cystectomy for bladder cancer.
Objectives: To compare recovery and morbidity after robot-assisted radical cystectomy with intracorporeal reconstruction vs open radical cystectomy.
Design, setting, and participants: Randomized clinical trial of patients with nonmetastatic bladder cancer recruited at 9 sites in the UK, from March 2017-March 2020. Follow-up was conducted at 90 days, 6 months, and 12 months, with final follow-up on September 23, 2021.
Interventions: Participants were randomized to receive robot-assisted radical cystectomy with intracorporeal reconstruction (n = 169) or open radical cystectomy (n = 169).
Main outcomes and measures: The primary outcome was the number of days alive and out of the hospital within 90 days of surgery. There were 20 secondary outcomes, including complications, quality of life, disability, stamina, activity levels, and survival. Analyses were adjusted for the type of diversion and center.
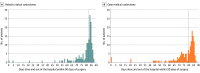
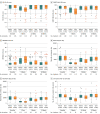
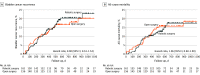
Results: Among 338 randomized participants, 317 underwent radical cystectomy (mean age, 69 years; 67 women [21%]; 107 [34%] received neoadjuvant chemotherapy; 282 [89%] underwent ileal conduit reconstruction); the primary outcome was analyzed in 305 (96%). The median number of days alive and out of the hospital within 90 days of surgery was 82 (IQR, 76-84) for patients undergoing robotic surgery vs 80 (IQR, 72-83) for open surgery (adjusted difference, 2.2 days [95% CI, 0.50-3.85]; P = .01). Thromboembolic complications (1.9% vs 8.3%; difference, -6.5% [95% CI, -11.4% to -1.4%]) and wound complications (5.6% vs 16.0%; difference, -11.7% [95% CI, -18.6% to -4.6%]) were less common with robotic surgery than open surgery. Participants undergoing open surgery reported worse quality of life vs robotic surgery at 5 weeks (difference in mean European Quality of Life 5-Dimension, 5-Level instrument scores, -0.07 [95% CI, -0.11 to -0.03]; P = .003) and greater disability at 5 weeks (difference in World Health Organization Disability Assessment Schedule 2.0 scores, 0.48 [95% CI, 0.15-0.73]; P = .003) and at 12 weeks (difference in WHODAS 2.0 scores, 0.38 [95% CI, 0.09-0.68]; P = .01); the differences were not significant after 12 weeks. There were no statistically significant differences in cancer recurrence (29/161 [18%] vs 25/156 [16%] after robotic and open surgery, respectively) and overall mortality (23/161 [14.3%] vs 23/156 [14.7%]), respectively) at median follow-up of 18.4 months (IQR, 12.8-21.1).
Conclusions and relevance: Among patients with nonmetastatic bladder cancer undergoing radical cystectomy, treatment with robot-assisted radical cystectomy with intracorporeal urinary diversion vs open radical cystectomy resulted in a statistically significant increase in days alive and out of the hospital over 90 days. However, the clinical importance of these findings remains uncertain.
Trial registration: ISRCTN Identifier: ISRCTN13680280; ClinicalTrials.gov Identifier: NCT03049410.
Conflict of interest statement
Figures
Comment in
-
Robotic Surgery for Bladder Cancer.JAMA. 2022 Jun 7;327(21):2085-2087. doi: 10.1001/jama.2022.6417. JAMA. 2022. PMID: 35569078 No abstract available.
-
Socioeconomic Factors, Urological Epidemiology and Practice Patterns.J Urol. 2022 Nov;208(5):1149-1150. doi: 10.1097/JU.0000000000002916. Epub 2022 Aug 17. J Urol. 2022. PMID: 35975570 No abstract available.
-
Effect of Robot-Assisted Radical Cystectomy vs Open Radical Cystectomy on 90-Day Morbidity and Mortality Among Patients With Bladder Cancer.JAMA. 2022 Sep 27;328(12):1257-1258. doi: 10.1001/jama.2022.13597. JAMA. 2022. PMID: 36166036 No abstract available.
-
Evolving the surgical approach for bladder cancer - robotic versus open radical cystectomy.Nat Rev Urol. 2023 Apr;20(4):203-204. doi: 10.1038/s41585-022-00704-z. Nat Rev Urol. 2023. PMID: 36522471 No abstract available.
References
-
- Jefferies ER, Cresswell J, McGrath JS, et al. ; BAUS Section on Oncology . Open radical cystectomy in England: the current standard of care—an analysis of the British Association of Urological Surgeons (BAUS) cystectomy audit and Hospital Episodes Statistics (HES) data. BJU Int. 2018;121(6):880-885. doi: 10.1111/bju.14143 - DOI - PubMed

