Sunflecks in the upper canopy: dynamics of light-use efficiency in sun and shade leaves of Fagus sylvatica
- PMID: 35569099
- PMCID: PMC9543657
- DOI: 10.1111/nph.18222
Sunflecks in the upper canopy: dynamics of light-use efficiency in sun and shade leaves of Fagus sylvatica
Abstract
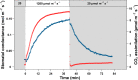
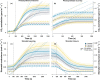
Sunflecks are transient patches of direct radiation that provide a substantial proportion of the daily irradiance to leaves in the lower canopy. In this position, faster photosynthetic induction would allow for higher sunfleck-use efficiency, as is commonly reported in the literature. Yet, when sunflecks are too few and far between, it may be more beneficial for shade leaves to prioritize efficient photosynthesis under shade. We investigated the temporal dynamics of photosynthetic induction, recovery under shade, and stomatal movement during a sunfleck, in sun and shade leaves of Fagus sylvatica from three provenances of contrasting origin. We found that shade leaves complete full induction in a shorter time than sun leaves, but that sun leaves respond faster than shade leaves due to their much larger amplitude of induction. The core-range provenance achieved faster stomatal opening in shade leaves, which may allow for better sunfleck-use efficiency in denser canopies and lower canopy positions. Our findings represent a paradigm shift for future research into light fluctuations in canopies, drawing attention to the ubiquitous importance of sunflecks for photosynthesis, not only in lower-canopy leaves where shade is prevalent, but particularly in the upper canopy where longer sunflecks are more common due to canopy openness.
Keywords: Fagus sylvatica; canopy vertical gradients; photosynthetic induction; provenance trial; stomatal dynamics; sun and shade leaves; sunfleck.
© 2022 The Authors. New Phytologist © 2022 New Phytologist Foundation.
Figures



References
-
- Allen JF, Bennett J, Steinback KE, Arntzen CJ. 1981. Chloroplast protein phosphorylation couples plastoquinone redox state to distribution of excitation energy between photosystems. Nature 291: 25–29.
-
- Allen MT, Pearcy RW. 2000. Stomatal versus biochemical limitations to dynamic photosynthetic performance in four tropical rainforest shrub species. Oecologia 122: 479–486. - PubMed
-
- Aphalo PJ, Ylianttila L 2021. R package: ooacquire v.0.2.3.9005: acquire data from OO spectrometers . [WWW document] URL https://docs.r4photobiology.info/ooacquire/, https://github.com/aphalo/ooacquire [accessed 1 June 2022].
MeSH terms
LinkOut - more resources
Full Text Sources

