MR imaging spectrum in COVID associated Rhino-Orbito-Cerebral mucormycosis with special emphasis on intracranial disease and impact on patient prognosis
- PMID: 35569303
- PMCID: PMC9074238
- DOI: 10.1016/j.ejrad.2022.110341
MR imaging spectrum in COVID associated Rhino-Orbito-Cerebral mucormycosis with special emphasis on intracranial disease and impact on patient prognosis
Abstract
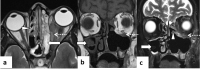
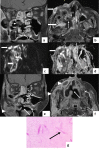
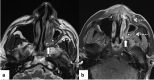
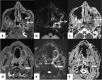
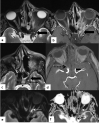
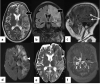
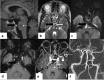
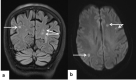
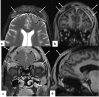
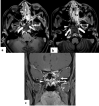
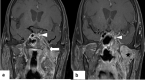
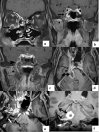
In the wake of the ongoing Coronavirus Disease 2019 (COVID-19) pandemic, a new epidemic of COVID associated mucormycosis (CAM) emerged in India. Early diagnosis and prompt treatment of this deadly disease are of paramount importance in improving patient survival. MRI is the cornerstone of diagnosis of early extrasinus disease, particularly intracranial complications which have traditionally been associated with a high mortality rate. In this review, we depict the sinonasal, perisinus, orbital and intracranial involvement in CAM. Special emphasis is laid on intracranial disease which is categorized into vascular, parenchymal, meningeal, bony involvement and perineural spread. Vascular complications are the most common form of intracranial involvement. Some unusual yet interesting imaging findings such as nerve abscesses involving the optic, trigeminal and mandibular nerves and long segment vasculitis of the internal carotid artery extending till its cervical segment are also illustrated. In our experience, patient outcome in CAM (survival rate of 88.5%) was better compared to the pre-pandemic era. Presence of intracranial disease also did not affect prognosis as poorly as traditionally expected (survival rate of 82.8%). Involvement of brain parenchyma was the only subset of intracranial involvement that was associated with higher mortality (p value 0.016). The aim of this review is to familiarise the reader with the MR imaging spectrum of CAM with special focus on intracranial complications and a brief account of their impact on patient prognosis in our experience.
Keywords: COVID associated mucormycosis; Fungal abscess; Intracranial complication; Invasive fungal sinusitis; Magnetic Resonance Imaging; Perineural spread; Rhinoorbitocerebral mucormycosis.
Copyright © 2022 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






Similar articles
-
COVID-19-Associated Rhino-Orbito-Cerebral Mucormycosis: A Single Tertiary Care Center Experience of Imaging Findings With a Special Focus on Intracranial Manifestations and Pathways of Intracranial Spread.Cureus. 2024 Apr 2;16(4):e57441. doi: 10.7759/cureus.57441. eCollection 2024 Apr. Cureus. 2024. PMID: 38699084 Free PMC article.
-
Magnetic Resonance Imaging in Coronavirus Disease - 2019 Associated Rhino-Orbital-Cerebral Mucormycosis (CA-ROCM) - Imaging Analysis of 50 Consecutive Patients.Curr Probl Diagn Radiol. 2022 Jan-Feb;51(1):112-120. doi: 10.1067/j.cpradiol.2021.09.004. Epub 2021 Nov 3. Curr Probl Diagn Radiol. 2022. PMID: 34802841 Free PMC article. Review.
-
MR Imaging in Covid-19-Associated Invasive Fungal Sinusitis.Neurol India. 2024 Sep 1;72(5):1009-1015. doi: 10.4103/neurol-india.ni_1225_21. Epub 2024 Oct 19. Neurol India. 2024. PMID: 39428773
-
Spectrum of intracranial complications of rhino-orbito-cerebral mucormycosis - resurgence in the era of COVID-19 pandemic: a pictorial essay.Emerg Radiol. 2021 Dec;28(6):1097-1106. doi: 10.1007/s10140-021-01987-2. Epub 2021 Oct 4. Emerg Radiol. 2021. PMID: 34605991 Free PMC article.
-
Imaging of COVID-19-associated craniofacial mucormycosis: a black and white review of the "black fungus".Clin Radiol. 2021 Nov;76(11):812-819. doi: 10.1016/j.crad.2021.07.004. Epub 2021 Jul 28. Clin Radiol. 2021. PMID: 34364672 Free PMC article. Review.
Cited by
-
Magnetic Resonance Imaging in COVID-19 Associated Rhino-Sinusal Mucormycosis.Curr Health Sci J. 2024 Jan-Mar;50(1):74-80. doi: 10.12865/CHSJ.50.01.10. Epub 2024 Mar 31. Curr Health Sci J. 2024. PMID: 38846483 Free PMC article.
-
Bacterial Brain Abscess and Life-Threatening Intracranial Hypertension Requiring Emergent Decompressive Craniectomy After SARS-CoV-2 Infection in a Healthy Adolescent.Cureus. 2023 Mar 16;15(3):e36258. doi: 10.7759/cureus.36258. eCollection 2023 Mar. Cureus. 2023. PMID: 37073194 Free PMC article.
-
COVID-19-Associated Rhino-Orbito-Cerebral Mucormycosis: A Single Tertiary Care Center Experience of Imaging Findings With a Special Focus on Intracranial Manifestations and Pathways of Intracranial Spread.Cureus. 2024 Apr 2;16(4):e57441. doi: 10.7759/cureus.57441. eCollection 2024 Apr. Cureus. 2024. PMID: 38699084 Free PMC article.
References
-
- Elinav H., Zimhony O., Cohen M.J., Marcovich A.L., Benenson S. Rhinocerebralmucormycosis in patients without predisposing medical conditions: a review of the literature. Clin. Microbiol. Infect. 2009;15(7):693–697. - PubMed
-
- Patel A., Kaur H., Xess I., Michael J.S., Savio J., Rudramurthy S., Singh R., Shastri P., Umabala P., Sardana R., Kindo A., Capoor M.R., Mohan S., Muthu V., Agarwal R., Chakrabarti A. A multicentre observational study on the epidemiology, risk factors, management and outcomes of mucormycosis in India. Clin. Microbiol. Infect. 2020;26(7) - PubMed
-
- Sen M., Honavar S.G., Bansal R., Sengupta S., Rao R., Kim U., et al. Epidemiology, clinical profile, management, and outcome of COVID–19–associated rhino–orbital–cerebral mucormycosis in 2826 patients in India – Collaborative OPAI–IJO Study on Mucormycosis in COVID–19 (COSMIC), Report 1. Indian J. Ophthalmol. 2021;69:1670–1692. - PMC - PubMed
-
- Meher R., Wadhwa V., Kumar V., Shisha Phanbuh D., Sharma R., Singh I., Rathore P.K., Goel R., Arora R., Garg S., Kumar S., Kumar J., Agarwal M., Singh M., Khurana N., Sagar T., Manchanda V., Saxena S. COVID associated mucormycosis: A preliminary study from a dedicated COVID Hospital in Delhi. Am. J. Otolaryngol. 2022;43(1):103220. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

