The addition of androgen deprivation therapy and pelvic lymph node treatment to prostate bed salvage radiotherapy (NRG Oncology/RTOG 0534 SPPORT): an international, multicentre, randomised phase 3 trial
- PMID: 35569466
- PMCID: PMC9819649
- DOI: 10.1016/S0140-6736(21)01790-6
The addition of androgen deprivation therapy and pelvic lymph node treatment to prostate bed salvage radiotherapy (NRG Oncology/RTOG 0534 SPPORT): an international, multicentre, randomised phase 3 trial
Abstract
Background: In men with a detectable prostate-specific antigen (PSA) level after prostatectomy for prostate cancer, salvage prostate bed radiotherapy (PBRT) results in about 70% of patients being free of progression at 5 years. A three-group randomised trial was designed to determine whether incremental gains in patient outcomes can be achieved by adding either 4-6 months of short-term androgen deprivation therapy (ADT) to PBRT, or both short-term ADT and pelvic lymph node radiotherapy (PLNRT) to PBRT.
Methods: The international, multicentre, randomised, controlled SPPORT trial was done at 283 radiation oncology cancer treatment centres in the USA, Canada, and Israel. Eligible patients (aged ≥18 years) were those who after prostatectomy for adenocarcinoma of the prostate had a persistently detectable or an initially undetectable and rising PSA of between 0·1 and 2·0 ng/mL. Patients with and without lymphadenectomy (N0/Nx) were eligible if there was no clinical or pathological evidence of lymph node involvement. Other eligibility criteria included pT2 or pT3 disease, prostatectomy Gleason score of 9 or less, and a Zubrod performance status of 0-1. Eligible patients were randomly assigned to receive PBRT alone at a dose of 64·8-70·2 Gy at 1·8 Gy per fraction daily (group 1), PBRT plus short-term ADT (group 2), or PLNRT (45 Gy at 1·8 Gy per fraction, and then a volume reduction made to the planning target volume for the remaining 19·8-25 ·2 Gy) plus PBRT plus short-term ADT (group 3). The primary endpoint was freedom from progression, in which progression was defined as biochemical failure according to the Phoenix definition (PSA ≥2 ng/mL over the nadir PSA), clinical failure (local, regional, or distant), or death from any cause. A planned interim analysis of 1191 patents with minimum potential follow-up time of 5 years applied a Haybittle-Peto boundary of p<0·001 (one sided) for comparison of 5-year freedom from progression rates between the treatment groups. This trial is registered with ClinicalTrials.gov, NCT00567580. The primary objectives of the trial have been completed, although long-term follow-up is continuing.
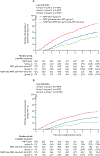
Findings: Between March 31, 2008, and March 30, 2015, 1792 eligible patients were enrolled and randomly assigned to the three treatment groups (592 to group 1 [PBRT alone], 602 to group 2 [PBRT plus short-term ADT], and 598 to group 3 [PLNRT plus PBRT plus short-term ADT]). 76 patients subsequently found to be ineligible were excluded from the analyses; thus, the evaluable patient population comprised 1716 patients. At the interim analysis (n=1191 patients; data cutoff May 23, 2018), the Haybittle-Peto boundary for 5-year freedom from progression was exceeded when group 1 was compared with group 3 (difference 17·9%, SE 2·9%; p<0·0001). The difference between groups 2 and 3 did not exceed the boundary (p=0·0063). With additional follow-up beyond the interim analysis (the final planned analysis; data cutoff May 26, 2021), at a median follow-up among survivors of 8·2 years (IQR 6·6-9·4), the 5-year freedom from progression rates in all 1716 eligible patients were 70·9% (95% CI 67·0-74·9) in group 1, 81·3% (78·0-84·6) in group 2, and 87·4% (84·7-90·2) in group 3. Per protocol criteria, freedom from progression in group 3 was superior to groups 1 and 2. Acute (≤3 months after radiotherapy) grade 2 or worse adverse events were significantly more common in group 3 (246 [44%] of 563 patients) than in group 2 (201 [36%] of 563; p=0·0034), which, in turn, were more common than in group 1 (98 [18%] of 547; p<0·0001). Similar findings were observed for grade 3 or worse adverse events. However, late toxicity (>3 months after radiotherapy) did not differ significantly between the groups, apart from more late grade 2 or worse blood or bone marrow events in group 3 versus group 2 (one-sided p=0·0060) attributable to the addition of PLNRT in this group.
Interpretation: The results of this randomised trial establish the benefit of adding short-term ADT to PBRT to prevent progression in prostate cancer. To our knowledge, these are the first such findings to show that extending salvage radiotherapy to treat the pelvic lymph nodes when combined with short-term ADT results in meaningful reductions in progression after prostatectomy in patients with prostate cancer.
Funding: National Cancer Institute.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Figures



Comment in
-
Combination improves salvage outcomes.Nat Rev Clin Oncol. 2022 Jul;19(7):428. doi: 10.1038/s41571-022-00651-z. Nat Rev Clin Oncol. 2022. PMID: 35606417 No abstract available.
-
ADT and node inclusion improves salvage radiation.Nat Rev Urol. 2022 Jul;19(7):384. doi: 10.1038/s41585-022-00614-0. Nat Rev Urol. 2022. PMID: 35672505 No abstract available.
-
Urological Oncology: Prostate Cancer.J Urol. 2022 Oct;208(4):931-934. doi: 10.1097/JU.0000000000002870. Epub 2022 Aug 16. J Urol. 2022. PMID: 35973210 No abstract available.
-
[Salvage prostate bed radiotherapy: co-irradiation of regional LNs and significance of ADT].Strahlenther Onkol. 2022 Dec;198(12):1119-1121. doi: 10.1007/s00066-022-02001-5. Epub 2022 Sep 9. Strahlenther Onkol. 2022. PMID: 36083310 Free PMC article. German. No abstract available.
-
The addition of pelvic lymph node treatment to prostate bed salvage radiotherapy.Lancet. 2022 Sep 17;400(10356):883-884. doi: 10.1016/S0140-6736(22)01440-4. Lancet. 2022. PMID: 36116475 No abstract available.
-
The addition of pelvic lymph node treatment to prostate bed salvage radiotherapy.Lancet. 2022 Sep 17;400(10356):884-885. doi: 10.1016/S0140-6736(22)01488-X. Lancet. 2022. PMID: 36116476 No abstract available.
-
The addition of pelvic lymph node treatment to prostate bed salvage radiotherapy.Lancet. 2022 Sep 17;400(10356):885. doi: 10.1016/S0140-6736(22)01482-9. Lancet. 2022. PMID: 36116478 No abstract available.
-
Re: The Addition of Androgen Deprivation Therapy and Pelvic Lymph Node Treatment to Prostate Bed Salvage Radiotherapy (NRG Oncology/RTOG 0534 SPPORT): An International, Multicentre, Randomised Phase 3 Trial.Eur Urol. 2023 May;83(5):475. doi: 10.1016/j.eururo.2022.12.026. Epub 2023 Jan 4. Eur Urol. 2023. PMID: 36609010 No abstract available.
References
-
- Bolla M, van Poppel H, Tombal B, et al. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911). Lancet 2012; 380(9858): 2018–27. - PubMed
-
- Wiegel T, Bartkowiak D, Bottke D, et al. Adjuvant radiotherapy versus wait-and-see after radical prostatectomy: 10-year follow-up of the ARO 96-02/AUO AP 09/95 trial. Eur Urol 2014; 66(2): 243–50. - PubMed
-
- Swanson GP, Hussey MA, Tangen CM, et al. Predominant treatment failure in postprostatectomy patients is local: analysis of patterns of treatment failure in SWOG 8794. J Clin Oncol 2007; 25(16): 2225–9. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

